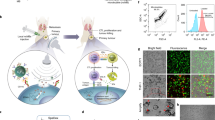
Abstract
One of the major limitations of cancer gene therapy using recombinant human adenovirus (Ad) is rapid Ad inactivation from systemic delivery. To eliminate this, biotin-coated ultrasound contrast agents, or microbubbles (MBs), were streptavidin-coupled with biotinylated antibodies to three distinct tumor vasculature-associated receptors (αVβ3 integrin, P-selectin and vascular endothelial growth factor receptor-2) for systemic targeting of a previously generated vector Ad5/3-Id1-SEAP-Id1-mCherry. This cancer-specific, dual-reporter vector was loaded in the targeted MBs and confirmed by confocal microscopy. MB loading capacity was estimated by functional assays as 4.72±0.2 plaque forming unit (PFU) per MB. Non-loaded (free) Ad particles were effectively inactivated by treatment with human complement. The Ad-loaded, targeted-MBs were injected systemically in mice bearing MDA-MB-231 tumors (Grp 1) and compared with two control groups: Ad-loaded, non-targeted MBs (Grp 2) and free Ad (Grp 3) administered under the same conditions. Two days after administration the blood levels of secreted embryonic alkaline phosphatase (SEAP) reporter in Grp 1 mice (16.1 ng ml–1±2.5) were significantly higher (P<0.05) than those in Grp 2 (9.75 ng ml–1±1.5) or Grp 3 (4.26 ng ml–1±2.5) animals. The targeted Ad delivery was also confirmed by fluorescence imaging. Thus, Ad delivery by targeted MBs holds potential as a safe and effective system for systemic Ad delivery for the purpose of cancer screening.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Du J, Li FH, Fang H, Xia JG, Zhu CX . Correlation of real-time gray scale contrast-enhanced ultrasonography with microvessel density and vascular endothelial growth factor expression for assessment of angiogenesis in breast lesions. J Ultrasound Med 2008; 27: 821–831.
Hudson JM, Karshafian R, Burns PN . Quantification of flow using ultrasound and microbubbles: a disruption replenishment model based on physical principles. Ultrasound Med Biol 2009; 35: 2007–2020.
Hoyt K, Warram JM, Umphrey H, Belt L, Lockhart ME, Robbin ML et al. Determination of breast cancer response to bevacizumab therapy using contrast-enhanced ultrasound and artificial neural networks. J Ultrasound Med 2010; 29: 577–585.
Guibal A, Taillade L, Mule S, Comperat E, Badachi Y, Golmard JL et al. Noninvasive contrast-enhanced US quantitative assessment of tumor microcirculation in a murine model: effect of discontinuing anti-VEGF therapy. Radiology 2010; 254: 420–429.
Tartis MS, McCallan J, Lum AF, LaBell R, Stieger SM, Matsunaga TO et al. Therapeutic effects of paclitaxel-containing ultrasound contrast agents. Ultrasound Med Biol 2006; 32: 1771–1780.
Ferrara K, Pollard R, Borden M . Ultrasound microbubble contrast agents: fundamentals and application to gene and drug delivery. Annu Rev Biomed Eng 2007; 9: 415–447.
Taylor SL, Rahim AA, Bush NL, Bamber JC, Porter CD . Targeted retroviral gene delivery using ultrasound. J Gene Med 2007; 9: 77–87.
Sorace AG, Warram JM, Umphrey H, Hoyt K . Microbubble-mediated ultrasonic techniques for improved chemotherapeutic delivery in cancer. J Drug Target 2012; 20: 43–54.
Hauff P, Seemann S, Reszka R, Schultze-Mosgau M, Reinhardt M, Buzasi T et al. Evaluation of gas-filled microparticles and sonoporation as gene delivery system: feasibility study in rodent tumor models. Radiology 2005; 236: 572–578.
Willmann JK, Deshpande RH, Lutz NAM, Cochran JR, Gambhir SS . Targeted contrast-enhanced ultrasound imaging of tumor angiogenesis with contrast microbubbles conjugated to integrin-binding knottin peptides. J Nucl Med 2010; 51: 433–440.
Klibanov AL . Microbubble contrast agents: targeted ultrasound imaging and ultrasound-assisted drug-delivery applications. Invest Radiol 2006; 41: 354–362.
Lindner JR . Microbubbles in medical imaging: current applications and future directions. Nat Rev Drug Discov 2004; 3: 527–532.
Klibanov AL . Ligand-carrying gas-filled microbubbles: ultrasound contrast agents for targeted molecular imaging. Bioconjugate Chem 2005; 16: 9–17.
Klibanov AL, Rychak JJ, Yang WC, Alikhani S, Li B, Acton S et al. Targeted ultrasound contrast agent for molecular imaging of inflammation in high-shear flow. Contrast Media Mol Imag 2006; 1: 259–266.
Behm CZ, Kaufmann BA, Carr C, Lankford M, Sanders JM, Rose CE et al. Molecular imaging of endothelial vascular cell adhesion molecule-1 expression and inflammatory cell recruitment during vasculogenesis and ischemia-mediated arteriogenesis. Circulation 2008; 117: 2902–2911.
Lindner JR . Contrast ultrasound molecular imaging of inflammation in cardiovascular disease. Cardiovasc Res 2009; 84: 182–189.
Kaufmann BA, Lindner JR . Molecular imaging with targeted contrast ultrasound. Curr Op in Biotech 2007; 18: 11–16.
Unger EC, McCreery TP, Sweitzer RH, Shen D, Wu G . In vitro studies of a new thrombus-specific ultrasound contrast agent. Am J Cardiol 1998; 81: 58–61.
Leong-Poi H, Christiansen J, Christiansen J, Klibanov AL, Kaul S, Lindner JR . Noninvasive assessment of angiogenesis by ultrasound microbubbles targeted to alpha-v integrins. Circulation 2003; 107: 455–460.
Weller GE, Wong MK, Modzelewski RA, Lu E, Klibanov AL, Wagner WR et al. Ultrasonic imaging of tumor angiogenesis using contrast microbubbles targeted via the tumor-binding peptide arginine-arginine-leucine. Cancer Res 2005; 65: 533–539.
Korpanty G, Carbon JG, Grayburn PA, Fleming JB, Brekken RA . Monitoring response to anticancer therapy by targeting microbubbles to tumor vasculature. Clin Cancer Res 2007; 13: 323.
Willmann JK, Lutz AM, Paulmurugan R, Patel MR, Chu P, Rosenberg J et al. Dual-targeted contrast agent for US assessment of tumor angiogenesis in vivo. Radiology 2008; 248: 936–944.
Weller GE, Villanueva FS, Tom EM, Wagner WR . Targeted ultrasound contrast agents: in vitro assessment of endothelial dysfunction and multi-targeting to ICAM-1 and sialyl Lewisx. Biotechnol Bioeng 2005; 92: 780–788.
Rapoport N, Gao Z, Kennedy A . Multifunctional nanoparticles for combining ultrasonic tumor imaging and targeted chemotherapy. J Natl Cancer Inst 2007; 99: 1095–1106.
Warram JM, Sorace AG, Saini R, Umphrey H, Zinn KR, Hoyt K . Triple-targeted US contrast agent provides improved localization to tumor vasculature. J Ultrasound Med 2011; 30: 921–931.
Lindner JR, Kaul S . Delivery of drugs with ultrasound. Echocardiography 2001; 18: 329–337.
Tinkov S, Bekeredjian R, Winter G, Coester C . Microbubbles as ultrasound triggered drug carriers. J Pharm Sci 2009; 98: 1935–1961.
Korpanty G, Chen S, Shohet RV, Ding J, Yang B, Frenkel PA et al. Targeting of VEGF-mediated angiogenesis to rat myocardium using ultrasonic destruction of microbubbles. Gene Ther 2005; 12: 1305–1312.
Tartis MS, McCallan J, Lum AF, LaBell R, Stieger SM, Matsunaga TO et al. Therapeutic effects of paclitaxel-containing ultrasound contrast agents. Ultrasound Med Biol 2006; 32: 1771–1780.
Unger EC, McCreery TP, Sweitzer RH, Caldwell VE, Wu Y . Acoustically active lipospheres containing paclitaxel: a new therapeutic ultrasound contrast agent. Invest Radiol 1998; 33: 886–892.
Qiu L, Zhang L, Wang L, Jiang Y, Luo Y, Peng Y et al. Ultrasound-targeted microbubble destruction enhances naked plasmid DNA transfection in rabbit Achilles tendons in vivo. Gene Ther; advance online publication, 10 October 2011 [E-pub ahead of print].
Mayer CR, Geis NA, Katus HA, Bekeredjian R . Ultrasound targeted microbubble destruction for drug and gene delivery. Expert Opin Drug Deliv 2008; 5: 1121–1138.
Howard CM, Forsberg F, Minimo C, Liu JB, Merton DA, Claudio PP . Ultrasound guided site-specific gene delivery system using adenoviral vectors and commercial ultrasound contrast agents. J Cell Physiol 2006; 209: 413–421.
Greco A, Di Benedetto A, Howard CM, Kelly S, Nande R, Dementieva Y et al. Eradication of therapy-resistant human prostate tumors using an ultrasound-guided site-specific cancer terminator virus delivery approach. Mol Ther 2010; 18: 295–306.
Smith RA, Cokkinides V, Brawley OW . Cancer screening in the United States, 2009: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2009; 59: 27–41.
Warram JM, Borovjagin AV, Zinn KR . A genetic strategy for combined screening and localized imaging of breast cancer. Mol Imaging Biol 2011; 13: 452–461.
Perk J, Iavarone A, Benezra R . Id family of helix-loop-helix proteins in cancer. Nat Rev Cancer 2005; 5: 603–614.
Borovjagin AV, Krendelchtchikov A, Ramesh N, Yu DC, Douglas JT, Curiel DT . Complex mosaicism is a novel approach to infectivity enhancement of adenovirus type 5-based vectors. Cancer Gene Ther 2005; 12: 475–486.
Kanerva A, Mikheeva GV, Krasnykh V, Coolidge CJ, Lam JT, Mahasreshti PJ et al. Targeting adenovirus to the serotype 3 receptor increases gene transfer efficiency to ovarian cancer cells. Clin Cancer Res 2002; 8: 275–280.
Matthews QL, Sibley DA, Wu H, Li J, Stoff-Khalili MA, Waehler R et al. Genetic incorporation of a herpes simplex virus type 1 thymidine kinase and firefly luciferase fusion into the adenovirus protein IX for functional display on the virion. Mol Imag 2006; 5: 510–519.
Le LP, Le HN, Dmitriev IP, Davydova JG, Gavrikova T, Yamamoto S et al. Dynamic monitoring of oncolytic adenovirus in vivo by genetic capsid labeling. J Natl Cancer Inst 2006; 98: 203–214.
Raper SE, Yudkoff M, Chirmule N, Gao GP, Nunes F, Haskal ZJ et al. A pilot study of in vivo liver-directed gene transfer with an adenoviral vector in partial ornithine transcarbamylase deficiency. Hum Gene Ther 2002; 13: 163–175.
Jakobsen JA, Oyen R, Thomsen HS, Morcos SKR . Members of Contrast Media Safety Committee of European Society of Urogenital. Safety of ultrasound contrast agents. Eur Radiol 2005; 15: 941–945.
Acknowledgements
This work was supported the UAB Small Animal Imaging Shared Facility NIH Research Core Grant (P30CA013148) and the Department of Defense (BC050034).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Warram, J., Sorace, A., Saini, R. et al. Systemic delivery of a breast cancer-detecting adenovirus using targeted microbubbles. Cancer Gene Ther 19, 545–552 (2012). https://doi.org/10.1038/cgt.2012.29
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2012.29
Keywords
This article is cited by
-
Synthetic biomarkers: a twenty-first century path to early cancer detection
Nature Reviews Cancer (2021)
-
A novel approach for assessment of prostate cancer aggressiveness using survivin-driven tumour-activatable minicircles
Gene Therapy (2019)
-
Microbubbles: from cancer detection to theranostics
Cancer Imaging (2015)
-
A dual-reporter, diagnostic vector for prostate cancer detection and tumor imaging
Gene Therapy (2014)
-
Antibody-based imaging strategies for cancer
Cancer and Metastasis Reviews (2014)