Abstract
The cytosolic innate immune sensor cyclic GMP-AMP synthase-stimulator of interferon genes (cGAS-STING) pathway is crucial for priming adaptive antitumour immunity through antigen-presenting cells (APCs). Natural agonists, such as cyclic dinucleotides (CDNs), activate the cGAS-STING pathway, but their clinical translation is impeded by poor cytosolic entry and serum stability, low specificity and rapid tissue clearance. Here we developed an ultrasound (US)-guided cancer immunotherapy platform using nanocomplexes composed of 2′3′-cyclic guanosine monophosphate-adenosine monophosphate (cGAMP) electrostatically bound to biocompatible branched cationic biopolymers that are conjugated onto APC-targeting microbubbles (MBs). The nanocomplex-conjugated MBs engaged with APCs and efficiently delivered cGAMP into the cytosol via sonoporation, resulting in activation of cGAS-STING and downstream proinflammatory pathways that efficiently prime antigen-specific T cells. This bridging of innate and adaptive immunity inhibited tumour growth in both localized and metastatic murine cancer models. Our findings demonstrate that targeted local activation of STING in APCs under spatiotemporal US stimulation results in systemic antitumour immunity and improves the therapeutic efficacy of checkpoint blockade, thus paving the way towards novel image-guided strategies for targeted immunotherapy of cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The authors declare that data supporting the findings of this study are available within the article and its Supplementary Information. The datasets generated and analysed during the study are publicly available at https://osf.io/phcmx. Source data are provided with this paper.
References
Jerby-Arnon, L. et al. A cancer cell program promotes T cell exclusion and resistance to checkpoint blockade. Cell 175, 984–997.e24 (2018).
Alsaiari, S. K. et al. Sustained and targeted delivery of checkpoint inhibitors by metal-organic frameworks for cancer immunotherapy. Sci. Adv. https://doi.org/10.1126/sciadv.abe7174 (2021).
Topalian, S. L., Taube, J. M. & Pardoll, D. M. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science https://doi.org/10.1126/science.aax0182 (2020).
Sun, X. et al. Amplifying STING activation by cyclic dinucleotide-manganese particles for local and systemic cancer metalloimmunotherapy. Nat. Nanotechnol. 16, 1260–1270 (2021).
Bendickova, K. & Fric, J. Roles of IL-2 in bridging adaptive and innate immunity, and as a tool for cellular immunotherapy. J. Leukoc. Biol. 108, 427–437 (2020).
Wen, L. et al. An efficient combination immunotherapy for primary liver cancer by harmonized activation of innate and adaptive immunity in mice. Hepatology 69, 2518–2532 (2019).
Lee, D., Huntoon, K., Wang, Y., Jiang, W. & Kim, B. Y. Harnessing innate immunity using biomaterials for cancer immunotherapy. Adv. Mater. https://doi.org/10.1002/adma.202007576 (2021).
Reislander, T., Groelly, F. J. & Tarsounas, M. DNA damage and cancer immunotherapy: a STING in the tale. Mol. Cell 80, 21–28 (2020).
Luo, M. et al. A STING-activating nanovaccine for cancer immunotherapy. Nat. Nanotechnol. 12, 648–654 (2017).
Shae, D. et al. Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat. Nanotechnol. 14, 269–278 (2019).
Cousin, C. et al. Persistence of integrase-deficient lentiviral vectors correlates with the induction of STING-independent CD8+ T cell responses. Cell Rep. 26, 1242–1257.e47 (2019).
Xu, N. et al. STING agonist promotes CAR T cell trafficking and persistence in breast cancer. J. Exp. Med. https://doi.org/10.1084/jem.20200844 (2021).
Miao, L. et al. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat. Biotechnol. 37, 1174–1185 (2019).
Ilovitsh, T. et al. Low-frequency ultrasound-mediated cytokine transfection enhances T cell recruitment at local and distant tumor sites. Proc. Natl Acad. Sci. USA 117, 12674–12685 (2020).
Feng, M. et al. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat. Rev. Cancer 19, 568–586 (2019).
Gutjahr, A. et al. The STING ligand cGAMP potentiates the efficacy of vaccine-induced CD8+ T cells. JCI Insight https://doi.org/10.1172/jci.insight.125107 (2019).
Chin, E. N. et al. Antitumor activity of a systemic STING-activating non-nucleotide cGAMP mimetic. Science 369, 993–999 (2020).
Decout, A., Katz, J. D., Venkatraman, S. & Ablasser, A. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat. Rev. Immunol. 21, 548–569 (2021).
Li, Y. et al. TMEM203 is a binding partner and regulator of STING-mediated inflammatory signaling in macrophages. Proc. Natl Acad. Sci. USA 116, 16479–16488 (2019).
Guo, Y. et al. Single-cell analysis reveals effective siRNA delivery in brain tumors with microbubble-enhanced ultrasound and cationic nanoparticles. Sci. Adv. https://doi.org/10.1126/sciadv.abf7390 (2021).
Dwivedi, P. et al. Magnetic targeting and ultrasound activation of liposome-microbubble conjugate for enhanced delivery of anticancer therapies. ACS Appl. Mater. Interfaces 12, 23737–23751 (2020).
Takahashi, M. et al. The tumor suppressor kinase DAPK3 drives tumor-intrinsic immunity through the STING-IFN-β pathway. Nat. Immunol. 22, 485–496 (2021).
Lahey, L. J. et al. LRRC8A:C/E heteromeric channels are ubiquitous transporters of cGAMP. Mol. Cell 80, 578–591.e5 (2020).
Ritchie, C., Cordova, A. F., Hess, G. T., Bassik, M. C. & Li, L. SLC19A1 is an importer of the immunotransmitter cGAMP. Mol. Cell 75, 372–381.e5 (2019).
Ahn, J., Xia, T., Capote, A. R., Betancourt, D. & Barber, G. N. Extrinsic phagocyte-dependent STING signaling dictates the immunogenicity of dying cells. Cancer Cell 33, 862–873.e5 (2018).
Li, X. et al. Lyn delivers bacteria to lysosomes for eradication through TLR2-initiated autophagy related phagocytosis. PLoS Pathog. 12, e1005363 (2016).
Yu, J., Deng, H. & Xu, Z. Targeting macrophage priming by polyphyllin VII triggers anti-tumor immunity via STING-governed cytotoxic T-cell infiltration in lung cancer. Sci. Rep. 10, 21360 (2020).
von Roemeling, C. A. et al. Therapeutic modulation of phagocytosis in glioblastoma can activate both innate and adaptive antitumour immunity. Nat. Commun. 11, 1508 (2020).
de Mingo Pulido, A. et al. The inhibitory receptor TIM-3 limits activation of the cGAS-STING pathway in intra-tumoral dendritic cells by suppressing extracellular DNA uptake. Immunity 54, 1154–1167.e7 (2021).
Lucas, E. D. et al. Type 1 IFN and PD-L1 coordinate lymphatic endothelial cell expansion and contraction during an inflammatory immune response. J. Immunol. 201, 1735–1747 (2018).
Ambler, R. et al. PD-1 suppresses the maintenance of cell couples between cytotoxic T cells and target tumor cells within the tumor. Sci. Signal. https://doi.org/10.1126/scisignal.aau4518 (2020).
Azzam, T. et al. Polysaccharide-oligoamine based conjugates for gene delivery. J. Med. Chem. 45, 1817–1824 (2002).
Lux, J. et al. Thrombin-activatable microbubbles as potential ultrasound contrast agents for the detection of acute thrombosis. ACS Appl. Mater. Interfaces 9, 37587–37596 (2017).
Assouvie, A., Daley-Bauer, L. P. & Rousselet, G. Growing murine bone marrow-derived macrophages. Methods Mol. Biol. 1784, 29–33 (2018).
Roney, K. Bone marrow-derived dendritic cells. Methods Mol. Biol. 1960, 57–62 (2019).
Underwood, W. & Anthony, R. AVMA Guidelines for the Euthanasia of Animals (AVMA, 2020).
Acknowledgements
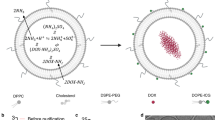
This work was supported in part by the Cancer Prevention and Research Institute of Texas (CPRIT) grants RR150010, RP210199 and RP19023, the Department of Defense grants W81XWH-21-1-0332/0333 and W81XWH-17-1-0401, the Susan G. Komen Foundation Career Catalyst Research grant CCR19605871 and the National Cancer Institute grant 1K08CA241070. R.F.M. is a CPRIT Established Investigator. Research reported in this publication was also supported by the Children’s Cancer Fund Comprehensive Center for Pediatric Oncology Research. The authors acknowledge Siemens Medical Solutions USA for the Siemens Sequoia ultrasound scanner loan. The authors acknowledge the UT Southwestern Harold C. Simmons Cancer Center Support grant P30 CA142543 for the support provided by the Small Animal Imaging shared resource. The authors also would like to acknowledge Erin Moore (Creatives Services, Department of Radiology, The University of Texas Southwestern Medical Center) for the illustrations in Fig. 1a,b and Supplementary Fig. 5a, and C. Wogan from the MD Anderson Cancer Center for editorial help.
Author information
Authors and Affiliations
Contributions
W.J. and J.L. conceived the project and were responsible for all phases of the research. X.L., S.K., Y.W., W.J. and J.L. designed the experiments. S.K., C.d.G.L., R.F.M. and J.L. conceived the microbubble platform. R.F.M. and J.L. provided guidance for ultrasound experiments. S.K. and N.N. prepared and characterized the MUSIC platform. X.L. and S.K. performed the in vitro experiments. X.L., S.K., M.Y. and J.S. performed the in vivo experiments. X.L. and S.K. collected the data. X.L., S.K., Y.W., K.H., D.L., Y.L., R.G., B.Y.S.K., W.J. and J.L. analysed and interpreted the data. X.L., S.K., Y.W., W.J. and J.L. performed the literature review. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
A provisional patent application based on the technology described in the manuscript has been filed by The University of Texas Southwestern Medical Center, with S.K., J.L. and W.J. as inventors, application number 63/173,956. All other authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Christopher Jewell and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–30 and Table 1.
Supplementary Video 1
cGAMP-loaded SpeDex MBs-aCD11b (ncMBs) were visualized using US during intratumoural injection.
Source data
Source Data Fig. 2
Unprocessed western blots.
Rights and permissions
About this article
Cite this article
Li, X., Khorsandi, S., Wang, Y. et al. Cancer immunotherapy based on image-guided STING activation by nucleotide nanocomplex-decorated ultrasound microbubbles. Nat. Nanotechnol. 17, 891–899 (2022). https://doi.org/10.1038/s41565-022-01134-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-022-01134-z
This article is cited by
-
Synthetic cationic helical polypeptides for the stimulation of antitumour innate immune pathways in antigen-presenting cells
Nature Biomedical Engineering (2024)
-
Overcoming acquired resistance to cancer immune checkpoint therapy: potential strategies based on molecular mechanisms
Cell & Bioscience (2023)
-
Focused ultrasound-mediated blood-brain barrier opening combined with magnetic targeting cytomembrane based biomimetic microbubbles for glioblastoma therapy
Journal of Nanobiotechnology (2023)
-
Targeting cGAS/STING signaling-mediated myeloid immune cell dysfunction in TIME
Journal of Biomedical Science (2023)
-
CAFs targeted ultrasound-responsive nanodroplets loaded V9302 and GLULsiRNA to inhibit melanoma growth via glutamine metabolic reprogramming and tumor microenvironment remodeling
Journal of Nanobiotechnology (2023)