Abstract
Oncolytic viruses are a promising method of cancer therapy, even for advanced malignancies. HF10, a spontaneously mutated herpes simplex type 1, is a potent oncolytic agent. The interaction of oncolytic herpes viruses with the tumor microenvironment has not been well characterized. We injected HF10 into tumors of patients with recurrent breast carcinoma, and sought to determine its effects on the tumor microenvironment. Six patients with recurrent breast cancer were recruited to the study. Tumors were divided into two groups: saline-injected (control) and HF10-injected (treatment). We investigated several parameters including neovascularization (CD31) and tumor lymphocyte infiltration (CD8, CD4), determined by immunohistochemistry, and apoptosis, determined by terminal deoxynucleotidyl transferase dUTP nick end labeling assay. Median apoptotic cell count was lower in the treatment group (P=0.016). Angiogenesis was significantly higher in treatment group (P=0.032). Count of CD8-positive lymphocytes infiltrating the tumors was higher in the treatment group (P=0.008). We were unable to determine CD4-positive lymphocyte infiltration. An effective oncolytic viral agent must replicate efficiently in tumor cells, leading to higher viral counts, in order to aid viral penetration. HF10 seems to meet this criterion; furthermore, it induces potent antitumor immunity. The increase in angiogenesis may be due to either viral replication or the inflammatory response.
Similar content being viewed by others
Introduction
Carcinoma of the breast is the most common cancer among females. Currently, 40% of breast cancer patients are predicted to suffer from either locoregional (isolated) recurrence or systemic metastasis. In all, 10–20% of all recurrences are locoregional, whereas 60–70% are distant metastases.1, 2, 3 Despite multimodal treatment, including chemotherapy and hormonal therapy, the prognosis for patients with recurrent or metastatic breast cancer remains poor.4 Therefore, more specific, safe and effective treatment modalities are required. A growing body of preclinical and clinical data suggests that oncolytic viral therapy could be an effective therapeutic modality in the treatment of advanced cancer.5, 6, 7, 8, 9, 10, 11
Various strains of viruses, such as adenovirus,12 herpes simplex virus,13 Newcastle disease virus, measles virus, vesicular stomatitis virus and vaccinia virus14 are being analyzed for their oncolytic capacity; some of these viruses have progressed to the clinical trial phase. Herpes simplex virus type 1 (HSV 1) is an ideal candidate for oncolytic viral therapy because of the following reasons: (a) it infects a broad range of hosts; (b) it causes lyses of the host cell at the end of viral replication; (c) it has a very large genome and therefore harbors many non-essential genes, mostly related to neuroinvasiveness that are expendable and can be replaced during the recombinant engineering process; (d) it can be controlled by antiviral drugs in the event of uncontrolled replication; and (e) its genome remains as an episome and does not incorporate in to the host genome, avoiding the risk of introducing mutations.15
A unique and spontaneously mutated and naturally mutated HSV 1, HF10, has been demonstrated to be an effective oncolytic agent in preclinical contexts including peritoneal dissemination models, breast cancer xenografts and malignant melanoma models.16, 17, 18, 19 In all these studies, HF10 has been effective in tumor lyses. Previously, we published a promising preliminary clinical study demonstrating the efficacy of HF10 in patients with recurrent breast cancers or unresectable pancreatic carcinoma.20, 21 The power of HF10 lies in the fact that it is a spontaneous mutant and is genetically very similar to the parental virus.
The tumor microenvironment has an important role in the survival, proliferation and invasion of tumor cells.22 The microenvironment is composed of non-transformed cells such as stromal, endothelial and immune cells, all of which are surrounded by the extracellular matrix.23 In order to increase in size, tumor tissue requires formation of new blood vessels nearby.24 Therefore, therapeutic modalities with an effect on angiogenesis are essential for successful cancer therapy. Preclinical data regarding herpetic stromal keratitis has revealed enhanced angiogenesis upon infection with wild-type HSV.25 The situation is more complicated for oncolytic viruses: some well-designed studies indicate that such viruses may have antiangiogenic properties,24, 26 whereas other researchers have reported enhanced angiogenesis occurring via a range of mechanisms.27, 28, 29 There are many attempts to engineer viruses to express antiangiogenic molecules.30, 31, 32 Also obscuring the true effect of oncolytic herpes viruses on angiogenesis is the fact that all data have been generated in animal models rather than clinical investigation.
Oncolytic viral therapy of cancer has an advantage when compared with conventional cancer therapeutics, namely, the potential to induce antitumor immunity due to viral replication and oncolysis. A number of preclinical studies have shown that oncolytic herpes viruses induce cytotoxic T lymphocyte-mediated antitumor immunity, which can inhibit tumor regrowth upon rechallenge.33, 34, 35, 36 Consistent with this, efforts have been made to arm oncolytic herpes viruses with cytokines such as interleukin-1237 and granulocyte-macrophage colony-stimulating factor10, 38 in order to intensify antitumor immunity. From this perspective, it might be said that the potency of a virus relies on its capacity to induce antitumor immunity.
In cells, induction of apoptosis can be a protective mechanism against viral infections.39 HSV 1 can reduce apoptosis via the ICP 34.5 gene product, which modifies the protein kinase R pathway, blocking the apoptotic mechanism in normal cells.39, 40, 41, 42 The effects of oncolytic herpes viruses on apoptosis are controversial: apoptosis is elevated upon viral treatment in some studies,43, 44 but reduced in others.45, 46 Some have argued that induction of premature apoptosis is not a desirable feature of oncolytic viral treatment;47 instead, reducing or delaying apoptosis may enhance viral penetration of the tumor, another determinant of the potency of an oncolytic virus.
The value of the data presented in this study relies on the fact that it was generated in actual clinical samples rather than experimental animal models. Here we aimed to evaluate the cellular effects of HSV 1, a spontaneous oncolytic mutant of HSV 1, on the tumor microenvironment in patients with recurrent carcinoma tumors.
Patients and methods
HF10 virus
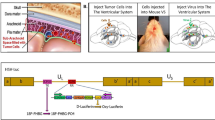
HF10 is a spontaneous mutant strain of HSV 1 whose mutations confine viral replication to cancer cells. The genome and genetic alterations of this herpes simplex virus are summarized in Figure 1.20 Briefly, the virus carries two main genetic alterations; (i) a 3832-bp deletion leading to loss of the UL56 promoter, making the gene dysfunctional; and (ii) near the terminal redundance sequence in long arm (TRL) end of the genome, a 6027-bp segment is present in an inverted orientation. The loss of UL56 function is the major functional alteration.16, 48 This mutation significantly decreases the neuroinvasiveness of the virus, possibly by decreasing axonal vesicular transport. Nevertheless; the mechanism that confines the virus to cancer cells is not clearly understood.
Summary of deletions and insertions in HF10’s genomic structure. The expansions indicate the position of genes within the deletion, insertions and relocalization. Arrows indicate the locations of the genes within expansions. bp, base pairs; IRS, internal repeat short; TRL, terminal repeat long; TRS, terminal repeat short; UL, unique long; US, unique short.
Patients’ characteristics
All the patients were female; their ages ranged from 48 to 76 years. All subjects were antibody-positive against HSV 1. Mastectomy had been performed on all of the patients, and all had received some treatment modality such as chemotherapy, endocrine therapy, surgical therapy and/or radiotherapy. Despite such treatment, however, these patients had recurrences and the disease had progressed to metastasis to the superficial or the subcutaneous region of the skin (Figure 2). The clinical parameters of the patients are summarized in Table 1. No specimen from patient number 6 was available, due to extensive fibrosis and a lack of tumor cells in the pathology sample. This study was approved by the local ethics committee and by the institutional review board of our hospital; all patients gave written informed consent.
Dosing interval of the virus
At least two tumor nodules were chosen; HF10 was injected into one nodule, and saline into the other as the mock control. The dosing regimen and the response to treatment for each patient are described in Table 2. In all patients, the first nodule (∼1 cm in diameter) was injected with virus suspended in diluents at various doses, as follows: single-dose injection of 104 plaque-forming units (pfu) per 0.5 ml to patient 1; single-dose injection of 105 pfu per 0.5 ml to patient 2; three-dose injection of 105 pfu per 0.5 ml to patient 3; single-dose injection of 5 × 105 pfu per 0.5 ml to patients 4 and 5; three-dose injection of 5 × 105 pfu per 0.5 ml to patient 6 (Table 2). Three or four different sites of the tumor were injected in order to infiltrate HF10 into the entire nodule. The second nodule, located more than 5 cm from the first nodule, was injected with 0.5 ml of sterile saline following the same dosing intervals as the viral injection counterpart. The tumors were resected 14 days following the initiation of treatment. Histopathological responses were evaluated according to criteria established by the Committee for Production of Histopathological Criteria of the Japanese Breast Cancer Society.49
Histological analysis
Standard hematoxylin−eosin staining was performed on 5-μm tissue sections for thorough histological analysis that included orientation of cells, herpetic inclusion bodies and general cellularity of the specimen under × 100 magnification.
Immunohistochemistry
Thick serial sections (5-μm) were taken. Antigen retrieval was carried out with Tris/EDTA, pH 9.0, in an autoclave for 15 min. After blocking with 3% normal goat serum (Histofine; Nichirei Biosciences, Tokyo, Japan), sections were incubated overnight with primary antibodies against CD8 and CD31 (dilution for both was 1:100) (Abcam, Cambridge, MA). Biotinylated antirabbit immunoglobulin G (Abcam) was used as the secondary antibody. HRP-DAB (Abcam) was used as the chromogenic agent, and hematoxylin as the counter-stain. Each slide was examined with a light microscope at × 200−400 magnification. Six random areas were chosen within each section, and the number of positive areas was counted for each area on each slide.
Colorimetric terminal deoxynucleotidyl transferase dUTP nick end labeling assay
Terminal deoxynucleotidyl transferase dUTP nick end labeling staining of tissue was carried out using a DeadEnd colorimetric apoptosis detection system, (Promega, Fitchburg, WI). Briefly, slides were immersed in 100 μl equilibration buffer at room temperature for 5–10 min. TdT reaction mix (Promega) was added onto the slides and incubated for 60 min at 37 °C in a humidified chamber. The reaction was stopped by immersing the slides in 2 × saline sodium citrate (Promega) for 15 min. Streptavidin HRP-DAB (Promega), diluted 1:500 in phosphate-buffered saline, was used as the chromogenic agent. Following the application of the cover slip, each slide was examined with a light microscope at × 100 magnification. In both control and HF10-treated groups; apoptotic cell counts were determined in six random areas in similar large tumor regions with moderate to high cell counts. Therefore the results were expressed as counts per high power field.
Anti-HSV 1 immunofluorescence
Polyclonal rabbit anti-herpes simplex virus type 1/FITC-conjugated antibody (DakoCytomation, Glostrup, Denmark) was used to perform the immunofluorescence staining according to the manufacturers’ instructions. Briefly, slides were deparaffinized, air dried and incubated with Polyclonal rabbit anti-herpes simplex virus type 1/FITC antibody (1:40 dilution) for 1 h. Subsequently, slides were air dried, mounted and observed with a fluorescence microscope. The slides were evaluated at × 100 magnification.
Statistical analysis
Data are expressed as means and s.d. values. The Mann−Whitney U-test was used to compare the data obtained from the study groups. Statistical analysis was performed using SPSS Statistics software, version 15.0 (SPSS, Chicago, IL). Statistically significant difference was inferred when P<0.05.
Results
Throughout the study; no adverse effects due to administration of HF10 were observed; all the patients tolerated the therapy without any problem.20
During the follow-up period, we observed a 30−70% reduction in the size of the tumors treated with HF10.
Microscopically, the tumors treated with HF10 showed lower cellularity than mock-treated control tumors (Figure 3). The cells gradually shrank and fibrosis took over. In fact, in the patient 6, the fibrosis was so massive that no tumor tissue could be identified; this sample was therefore excluded from the histopathological analysis. HSV 1 inclusion bodies could be observed in the HF10-treated group; anti-HSV 1 immunofluorescence staining also confirmed the presence of the virus (Figure 4a). HSV antigen was detected at the tumor islands and not in normal tissue. Furthermore; the viral antigen was distributed throughout the tumor. The mock-treated control tumors did not express the antigen.
(a) Herpetic inclusion bodies (arrow) are shown, as well as immunofluorescence staining. (b) The apoptotic cell count was reduced in the HF10-treated tumors (arrows). (c) CD31-positive microvascular density is enhanced in the HF10-treated tumors. (d) At closer magnification, it is clear that in some areas the neovascularization extended into the tumor islets (arrows). (e) CD8-positive lymphocyte infiltration is observed higher in tumors treated with HF10 than in the mock-treated tumors. (f) CD4-positive cells were not detected in any group. TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
Mean apoptotic cell count was 25.6 per high power field (hpf) at × 100 magnification in HF10-treated tumors, vs 47.4 per hpf in the control tumors (Figure 4b, P=0.016). The apoptotic bodies were scattered uniformly throughout the tumor islets.
As shown in Figure 4c, neovascularization (identified by CD31 staining) was significantly higher in HF10-treated tumors than in control tumors (mean of 30.0 per hpf at × 200 magnification, vs 12.0 per hpf; P=0.032). The neovascularization areas were more prominent at the junction between tumor and stroma. At higher magnification, we observed that some regions contained extensions into the tumor islets (Figure 4d).
Mean counts of CD8-positive lymphocytes infiltrating the tumors in the HF10-treated and control tumors were 75.0 and 42.0 per hpf at × 400 magnification, respectively (Figure 4e, P=0.008). CD8-positive T lymphocytes surrounded each tumor islet; as they infiltrated more deeply, the tumor cells became more hyperchromatic and shrunken in size, suggestive of a cytotoxic T cell-mediated antitumoral immune response. We were unable to detect CD4-positive cells in either HF10-treated or mock-treated tumors (Figure 4f).
Discussion
Cancer is a complex and a multi-factorial disease. Following malignant transformation, cancer cells need an environment that is suitable for nourishment, proliferation, migration and invasion. The tumor microenvironment is very similar to sites of inflammation during the wound healing process, which promotes angiogenesis, turnover of the extracellular matrix and tumor cell motility.50 The tumor microenvironment consists of non-malignant cellular components such as fibroblasts, endothelial cells and immune cells. Therefore, in order to elucidate the mechanism of tumor lysis by oncolytic viral agents, it is important to understand the interaction between the viruses and the tumor microenvironment.
HF10 is a spontaneously occurring mutant virus isolated from the herpes simplex type 1 strain HF by Nishiyama et al.51 The antitumor effects of HF10 are more potent than those of genetically engineered viruses, because HF10 is a spontaneously mutated virus. Our preclinical and preliminary clinical data suggest that HF10 is an effective agent for treatment of non-neurogenic tumors.52 Before now, however, the effects of HF10 and other oncolytic herpes viruses on cellular components of the tumor microenvironment have not been well characterized. This is the first study to specifically analyze the effects of this virus on the tumor microenvironment following intratumoral injection in patients with recurrent breast cancer.
In this study, we observed that HF10-treated tumors had significantly lower apoptotic cell counts than mock-treated tumors. We believe that HSV 1 has a tendency to reduce apoptosis through several mechanisms, such as the PKR pathway and the US3 arm of the viral genome.53 Some oncolytic herpes viruses reduce apoptosis;45, 46, 54 whereas others enhance apoptosis.43, 44, 47 The effect of oncolytic herpes viruses on apoptosis is strain specific and depends on the underlying genetic variation. Defects in the US3 arm or gamma 34.5 may induce apoptosis in infected cells; this may explain why gamma 34.5 deleted viruses such as G207 induce apoptosis. In such cases, the virus usually has a limited infection area and cannot spread throughout the tumor. In contrast to G207, HF10 spread throughout the whole tumor area, as demonstrated by staining for the HSV1 antigen. The fact that HF10 reduces apoptosis may be related to its ability to spread throughout the tumor instead of being confined only to the injection site. Eisenberg et al.55 reported that virus-related apoptosis in pancreatic cancer cell lines, which is induced by Hsp72, was reduced in hyperthermia; if virus-related apoptosis is reduced, in vitro viral titers and cytotoxicity will be increased. These observations suggest that if viral infection causes apoptosis, the amount of infectious particles will gradually decrease. In a study of E1B55 attenuated adenovirus, Ganly et al.56 emphasized that virus-induced apoptosis was distinct from virus-induced cytolysis: apoptosis causes a premature cessation of viral replication, whereas cytolysis results in release of infective progeny.45 In the present study; it may be hypothesized that lower cell counts in the HF10-treated group have caused the low apoptosis counts. However; we selected similar tumor areas with moderate to high cell number; for both control and HF10-treated groups; and performed the cell counts in six random fields in the same area. Therefore, we believe that our results in fact show that apoptosis is reduced by HF10.
We also observed that oncolytic viral therapy with HF10 enhanced angiogenesis, possibly due to the inflammatory response induced by viral infection, and viral proteins expressed during viral replication.30 The mechanisms underlying the enhanced inflammation are not precisely known, but preclinical data regarding herpetic stromal keratitis in wild-type HSV 1 infection revealed that angiogenesis may be induced by paracrine effects resulting from release of VP22 or the CpG motifs in the DNA of HSV 1, which are required for viral replication.57, 58, 59, 60 Most of these stimuli, especially the CpG motif, potently stimulate secretion of vascular endothelial growth factor (VEGF) A.58, 59, 60, 61 In addition, antiangiogenic molecules such as thrombospondin 1 and 2 are reduced in wild-type HSV 1 infection.59 Many studies have shown that VEGF A is upregulated in HSV 1 infections;62, 63, 64 thus, VEGF A may be the factor underlying the angiogenesis seen in our study.
Angiogenesis induces tumorigenesis. One study by Florence et al.65 showed that angiogenesis is higher in invasive cutaneous squamous cell cancers than in carcinoma in situ or microinvasive carcinoma. In addition, a number of studies have shown beneficial effects of antiangiogenic treatment in various cancer models.66, 67, 68, 69 The angiogenesis caused by oncolytic herpes viruses may have deleterious effects on the late phase of cancer therapy, especially with regard to late recurrences that occur after the end of the treatment regimen. In order to investigate this effect, we initiated a series of preclinical studies combining oncolytic herpes viruses with the monoclonal anti-VEGFA antibody bevacizumab in various cancer models.
Our results suggest that treatment with HF10 induces a cytotoxic T lymphocyte response directed against the tumor. This has been supported by many studies of oncolytic viruses including HF10.17, 19, 33, 34, 35 Furthermore, HF10 induces antitumor immunity more efficiently than hrR3, which is also an HSV 1 variant.17 Oncolytic replication of a virus is an immunogenic event69 that generates a response against both viral and tumor antigens.70, 71 Herpes simplex viruses induce antitumor immunity by activation of dendritic cells via Toll-like receptors 2 and 9, which in turn enhance antigen presentation and specific T and B lymphocyte responses.72, 73, 74 In addition, herpes simplex virus reduces the number of myeloid-derived suppressor cells, which contribute to tumor cells’ ability to circumvent host immune surveillance.61 This effect is possibly due to reducing the effects of induced the expression of VEGF A on vascular endothelial growth factor receptor 2 through soluble neuropilin-1.61 Another explanation of the enhanced immune response may be the syncytial cytopathic effect induced by HF10, which is a very potent immune enhancer.52, 75, 76 Today we are certain that every oncolytic virus induces a certain level of antitumor immune response, yet the potency of the response determines the efficacy of the virus.
In summary, the clinical data obtained in this study show that HF10 is a powerful oncolytic virus. By reducing apoptosis, it can thoroughly penetrate the tumor; furthermore, it induces a potent antitumor immune response that results in an efficient reduction of tumor volume. Its enhanced oncolytic activity is owed in part to the fact that it is a spontaneous, rather than engineered, mutant. All of the aforementioned factors, and the cytopathic effect of the virus contribute to the potent antitumor immunity caused by HF10. These characteristics make HF10 a potent, safe, promising oncolytic agent for the treatment of advanced carcinoma.
References
Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A et al. Cancer statistics, 2005. CA Cancer J Clin 2005; 55: 10–30.
Jemal A, Thun MJ, Ries LA, Howe HL, Weir HK, Center MM et al. Annual report to the nation on the status of cancer, 1975–2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst 2008; 100: 1672–1694.
Kamby C, Ejlertsen B, Andersen J, Birkler NE, Rytter L, Zedeler K et al. The pattern of metastases in human breast cancer. Influence of systemic adjuvant therapy and impact on survival. Acta Oncol 1988; 27: 715–719.
Gerber B, Freund M, Reimer T . Recurrent breast cancer: treatment strategies for maintaining and prolonging good quality of life. Dtsch Arztebl Int 2010; 107: 85–91.
Nomura N, Kasuya H, Watanabe I, Shikano T, Shirota T, Misawa M et al. Considerations for intravascular administration of oncolytic herpes virus for the treatment of multiple liver metastases. Cancer Chemother Pharmacol 2009; 63: 321–330.
Wong RJ, Kim SH, Joe JK, Shah JP, Johnson PA, Fong Y . Effective treatment of head and neck squamous cell carcinoma by an oncolytic herpes simplex virus. J Am Coll Surg 2001; 193: 12–21.
Bennett JJ, Delman KA, Burt BM, Mariotti A, Malhotra S, Zager J et al. Comparison of safety, delivery, and efficacy of two oncolytic herpes viruses (G207 and NV1020) for peritoneal cancer. Cancer Gene Ther 2002; 9: 935–945.
Cinatl Jr J, Cinatl J, Michaelis M, Kabickova H, Kotchetkov R, Vogel JU et al. Potent oncolytic activity of multimutated herpes simplex virus G207 in combination with vincristine against human rhabdomyosarcoma. Cancer Res 2003; 63: 1508–1514.
Kubo S, Kawasaki Y, Yamaoka N, Tagawa M, Kasahara N, Terada N et al. Complete regression of human malignant mesothelioma xenografts following local injection of midkine promoter-driven oncolytic adenovirus. J Gene Med 2010; 12: 681–692.
Harrington KJ, Hingorani M, Tanay MA, Hickey J, Bhide SA, Clarke PM et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin Cancer Res 2010; 16: 4005–4015.
Watanabe I, Kasuya H, Nomura N, Shikano T, Shirota T, Kanazumi N et al. Effects of tumor selective replication-competent herpes viruses in combination with gemcitabine on pancreatic cancer. Cancer Chemother Pharmacol 2008; 61: 875–882.
Guse K, Hemminki A . Cancer gene therapy with oncolytic adenoviruses. J BUON 2009; 14: 7–15.
Watanabe D . Medical application of herpes simplex virus. J Dermatol Sci 2010; 57: 75–82.
Haseley A, Alvarez-Breckenridge C, Chaudhury AR, Kaur B . Advances in oncolytic virus therapy for glioma. Recent Pat CNS Drug Discov 2009; 4: 1–13.
Varghese S, Rabkin SD . Oncolytic herpes simplex virus vectors for cancer virotherapy. Cancer Gene Ther 2002; 9: 967–978.
Nawa A, Luo C, Zhang L, Ushjima Y, Ishida D, Kamakura M et al. Non-engineered, naturally oncolytic herpes simplex virus HSV1 HF-10: applications for cancer gene therapy. Curr Gene Ther 2008; 8: 208–221.
Shimoyama S, Goshima F, Teshigahara O, Kasuya H, Kodera Y, Nakao A et al. Enhanced efficacy of herpes simplex virus mutant HF10 combined with paclitaxel in peritoneal cancer dissemination models. Hepatogastroenterology 2007; 54: 1038–1042.
Teshigahara O, Goshima F, Takao K, Kohno S, Kimata H, Nakao A et al. Oncolytic viral therapy for breast cancer with herpes simplex virus type 1 mutant HF10. J Surg Oncol 2004; 85: 42–47.
Watanabe D, Goshima F, Mori I, Tamada Y, Matsumoto Y, Nishiyama Y . Oncolytic virotherapy for malignant melanoma with herpes simplex virus type 1 mutant HF10. J Dermatol Sci 2008; 50: 185–196.
Kimata H, Imai T, Kikumori T, Teshigahara O, Nagasaka T, Goshima F et al. Pilot study of oncolytic viral therapy using mutant herpes simplex virus (HF10) against recurrent metastatic breast cancer. Ann Surg Oncol 2006; 13: 1078–1084.
Nakao A, Takeda S, Shimoyama S, Kasuya H, Kimata H, Teshigahara O et al. Clinical experiment of mutant herpes simplex virus HF10 therapy for cancer. Curr Cancer Drug Targets 2007; 7: 169–174.
Marx J . Cancer biology. All in the stroma: cancer's Cosa Nostra. Science 2008; 320: 38–41.
Wojton J, Kaur B . Impact of tumor microenvironment on oncolytic viral therapy. Cytokine Growth Factor Rev 2010; 21: 127–134.
Benencia F, Courreges MC, Conejo-García JR, Buckanovich RJ, Zhang L, Carroll RH et al. Oncolytic HSV exerts direct antiangiogenic activity in ovarian carcinoma. Hum Gene Ther 2005; 16: 765–778.
Zheng M, Deshpande S, Lee S, Ferrara N, Rouse BT . Contribution of vascular bendothelial growth factor in the neovascularization process during thepathogenesis of herpetic stromal keratitis. J Virol 2001; 75: 9828–9835.
Cinatl Jr J, Michaelis M, Driever PH, Cinatl J, Hrabeta J, Suhan T et al. Multimutated herpes simplex virus G207 is a potent inhibitor of angiogenesis. Neoplasia 2004; 6: 725–735.
Aghi M, Rabkin SD, Martuza RL . Angiogenic response caused by oncolyticherpes simplex virus-induced reduced thrombospondin expression can be prevented by specific viral mutations or by administering a thrombospondin derived peptide. Cancer Res 2007; 67: 440–444.
Kurozumi K, Hardcastle J, Thakur R, Shroll J, Nowicki M, Otsuki A et al. Oncolytic HSV-1 infection of tumors induces angiogenesis and upregulates CYR61. Mol Ther 2008; 16: 1382–1391.
Wong RJ, Chan MK, Yu Z, Ghossein RA, Ngai I, Adusumilli PS et al. Angiogenesis inhibition by an oncolytic herpes virus expressing interleukin 12. Clin Cancer Res 2004; 10: 4509–4516.
Hardcastle J, Kurozumi K, Dmitrieva N, Sayers MP, Ahmad S, Waterman P et al. Enhanced antitumor efficacy of vasculostatin (Vstat120) expressing oncolytic HSV-1. Mol Ther 2010; 18: 285–2894.
Liu TC, Zhang T, Fukuhara H, Kuroda T, Todo T, Martuza RL et al. Oncolytic HSV armed with platelet factor 4, an antiangiogenic agent, shows enhanced efficacy. Mol Ther 2006; 14: 789–797.
Mullen JT, Donahue JM, Chandrasekhar S, Yoon SS, Liu W, Ellis LM et al. Oncolysis by viral replication and inhibition of angiogenesis by a replication-conditional herpes simplex virus that expresses mouse endostatin. Cancer 2004; 101: 869–877.
Todo T, Rabkin SD, Sundaresan P, Wu A, Meehan KR, Herscowitz HB et al. Systemic antitumor immunity in experimental brain tumor therapy using amultimutated, replication-competent herpes simplex virus. Hum Gene Ther 1999; 10: 2741–2755.
Todo T, Martuza RL, Rabkin SD, Johnson PA . Oncolytic herpes simplex virüs vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci USA 2001; 98: 6396–6401.
Todo T, Rabkin SD, Chahlavi A, Martuza RL . Corticosteroid administration does not affect viral oncolytic activity, but inhibits antitumor immunity in replication-competent herpes simplex virus tumor therapy. Hum Gene Ther 1999; 10: 2869–2878.
Benencia F, Courrèges MC, Conejo-García JR, Mohamed-Hadley A, Zhang L, Buckanovich RJ et al. HSV oncolytic therapy upregulates interferon-inducible chemokines and recruits immune effector cells in ovarian cancer. Mol Ther 2005; 12: 789–802.
Bennett JJ, Malhotra S, Wong RJ, Delman K, Zager J, St-Louis M et al. Interleukin 12 secretion enhances antitumor efficacy of oncolytic herpes simplex viral therapy for colorectal cancer. Ann Surg 2001; 233: 819–826.
Kaufman HL, Kim DW, DeRaffele G, Mitcham J, Coffin RS, Kim-Schulze S . Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann Surg Oncol 2010; 17: 718–730.
Koyama AH, Fukumori T, Fujita M, Irie H, Adachi A . Physiological significance of apoptosis in animal virus infection. Microbes Infect 2000; 2: 1111–1117.
Lan P, Dong C, Qi Y, Xiao G, Xue F . Gene therapy for mice sarcoma with oncolytic herpes simplex virus-1 lacking the apoptosis-inhibiting gene, icp34.5. J Biochem Mol Biol 2003; 36: 379–386.
Wong HH, Lemoine NR, Wang Y . Oncolytic viruses for cancer therapy: overcoming the obstacles. Viruses 2010; 2: 78–106.
He B, Gross M, Roizman B . The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc Natl Acad Sci USA 1997; 94: 843–848.
Todryk S, McLean C, Ali S, Entwistle C, Boursnell M, Rees R et al. Disabled infectious single-cycle herpes simplex virus as an oncolytic vector for immunotherapy of colorectal cancer. Hum Gene Ther 1999; 10: 2757–2768.
Spear MA, Sun F, Eling DJ, Gilpin E, Kipps TJ, Chiocca EA et al. Cytotoxicity, apoptosis, and viral replication in tumor cells treated withoncolytic ribonucleotide reductase-defective herpes simplex type 1 virus (hrR3)combined with ionizing radiation. Cancer Gene Ther 2000; 7: 1051–1059.
Passer BJ, Castelo-Branco P, Buhrman JS, Varghese S, Rabkin SD, Martuza RL . Oncolytic herpes simplex virus vectors and taxanes synergize to promote killing of prostate cancer cells. Cancer Gene Ther 2009; 16: 551–560.
Lin SF, Gao SP, Price DL, Li S, Chou TC, Singh P et al. Synergy of a herpes oncolytic virus and paclitaxel for anaplastic thyroid cancer. Clin Cancer Res 2008; 14: 1519–1528.
Huszthy PC, Immervoll H, Wang J, Goplen D, Miletic H, Eide GE et al. Cellular effects of oncolytic viral therapy on the glioblastoma microenvironment. Gene Ther 2010; 17: 202–216.
Mori I, Nishiyama Y . Accessory genes define the relationship between the herpes simplex virus and its host. Microbes Infect 2006; 8: 2556–2562.
Committee for Production of Histopathological Criteria, Japanese Breast Cancer Society. Histopathological criteria for assessment of therapeutic response in breast cancer. Breast Cancer 2001; 8: 1–7.
Kessenbrock K, Plaks V, Werb Z . Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 2010; 141: 52–67.
Nishiyama Y, Kimura H, Daikoku T . Complementary lethal invasion of the central nervous system by nonneuroinvasive herpes simplex virus types 1 and 2. J Virol 1991; 65: 4520–4524.
Ushijima Y, Luo C, Goshima F, Yamauchi Y, Kimura H, Nishiyama Y . Determination and analysis of the DNA sequence of highly attenuated herpes simplex virus type 1 mutant HF10, a potential oncolytic virus. Microbes Infect 2007; 9: 142–149.
Liu TC, Wakimoto H, Martuza RL, Rabkin SD . Herpes simplex virus Us3(-) mutant as oncolytic strategy and synergizes with phosphatidylinositol 3-kinase-Akt targeting molecular therapeutics. Clin Cancer Res 2007; 13: 5897–5902.
Dai MH, Zamarin D, Gao SP, Chou TC, Gonzalez L, Lin SF et al. Synergistic action of oncolytic herpes simplex virus and radiotherapy in pancreatic cancercell lines. Br J Surg 2010; 97: 1385–1394.
Eisenberg DP, Carpenter SG, Adusumilli PS, Chan MK, Hendershott KJ, Yu Z et al. Hyperthermia potentiates oncolytic herpes viral killing of pancreatic cancer through a heat shock protein pathway. Surgery 2010; 148: 325–334.
Ganly I, Kim YT, Hann B, Balmain A, Brown R . Replication and cytolysis of anE1B-attenuated adenovirus in drug-resistant ovarian tumour cells is associated with reduced apoptosis. Gene Ther 2001; 8: 369–375.
Zheng M, Schwarz MA, Lee S, Kumaraguru U, Rouse BT . Control of stromal keratitis by inhibition of neovascularization. Am J Pathol 2001; 159: 1021–1029.
Zheng M, Klinman DM, Gierynska M, Rouse BT . DNA containing CpG motifs induces angiogenesis. Proc Natl Acad Sci USA 2002; 99: 8944–8949.
Choudhary A, Hiscott P, Hart CA, Kaye SB, Batterbury M, Grierson I . Suppression of thrombospondin 1 and 2 production by herpes simplex virus 1 infection in cultured keratocytes. Mol Vis 2005; 11: 163–168.
Wuest TR, Carr DJ . VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis. J Exp Med 2010; 207: 101–115.
Ohkusu-Tsukada K, Ohta S, Kawakami Y, Toda M . Adjuvant effects of formalin-inactivated HSV through activation of dendritic cells and inactivation of myeloid-derived suppressor cells in cancer immunotherapy. Int J Cancer 2011; 128: 119–131.
Boshoff C . Kaposi's sarcoma. Coupling herpesvirus to angiogenesis. Nature 1998; 391: 24–25.
Hayashi K, Hooper LC, Detrick B, Hooks JJ . HSV immune complex (HSV-IgG: IC) and HSV-DNA elicit the production of proangiogenic factors such as VEGF and MMP-9. Arch Virol 2009; 154: 219–226.
Hosseini H, Khalili MR . Therapeutic potential of bevacizumab (Avastin) in herpetic stromal keratitis (HSK). Med Hypotheses 2007; 69: 568–570.
Florence ME, Massuda JY, Bröcker EB, Metze K, Cintra ML, Souza EM . Angiogenesis in the progression of cutaneous squamous cell carcinoma: an immunohistochemical study of endothelial markers. Clinics (Sao Paulo) 2011; 66: 465–468.
Cerullo V, Pesonen S, Diaconu I, Escutenaire S, Arstila PT, Ugolini M et al. Oncolytic adenovirus coding for granulocyte macrophage colony-stimulating factor induces antitumoral immunity in cancer patients. Cancer Res 2010; 70: 4297–4309.
Song KS, Li G, Kim JS, Jing K, Kim TD, Kim JP et al. Protein-bound polysaccharide from Phellinus linteus inhibits tumor growth, invasion, and angiogenesis and alters Wnt/beta-catenin in SW480 human colon cancer cells. BMC Cancer 2011; 11: 307–317.
Ma J, Chen CS, Blute T, Waxman DJ . Antiangiogenesis enhances intratumoral drug retention. Cancer Res 2011; 71: 2675–2685.
Bergers G, Javaherian K, Lo KM, Folkman J, Hanahan D . Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science 1999; 284: 808–812.
Lou E . Oncolytic herpes viruses as a potential mechanism for cancer therapy. Acta Oncol 2003; 42: 660–671.
Barba D, Hardin J, Sadelain M, Gage FH . Development of antitumor immunity following thymidine kinase-mediated killing of experimental brain tumors. Proc Natl Acad Sci USA 1994; 91: 4348–4352.
Kurt-Jones EA, Chan M, Zhou S, Wang J, Reed G, Bronson R et al. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc Natl Acad Sci USA 2004; 101: 1315–1320.
Krug A, Luker GD, Barchet W, Leib DA, Akira S, Colonna M . Herpes simplex virustype 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood 2004; 103: 1433–1437.
Reske A, Pollara G, Krummenacher C, Katz DR, Chain BM . Glycoprotein-dependent and TLR2-independent innate immune recognition of herpes simplex virus-1 by dendritic cells. J Immunol 2008; 180: 7525–7536.
Li H, Dutuor A, Tao L, Fu X, Zhang X . Virotherapy with a type 2 herpes simplex virus-derived oncolytic virus induces potent antitumor immunity against neuroblastoma. Clin Cancer Res 2007; 13: 316–322.
Nakamori M, Fu X, Rousseau R, Chen SY, Zhang X . Destruction of nonimmunogenic mammary tumor cells by a fusogenic oncolytic herpes simplex virus induces potent antitumor immunity. Mol Ther 2004; 9: 658–665.
Acknowledgements
This study has been supported by the Takeda Science Foundation 2008 Grant-in-Aid for Science Research in Japan, the Ichihara International Scholar Foundation and the Nitto Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Sahin, T., Kasuya, H., Nomura, N. et al. Impact of novel oncolytic virus HF10 on cellular components of the tumor microenviroment in patients with recurrent breast cancer. Cancer Gene Ther 19, 229–237 (2012). https://doi.org/10.1038/cgt.2011.80
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2011.80
Keywords
This article is cited by
-
Oncolytic HSV1 targets different growth phases of breast cancer leptomeningeal metastases
Cancer Gene Therapy (2023)
-
Advances and potential pitfalls of oncolytic viruses expressing immunomodulatory transgene therapy for malignant gliomas
Cell Death & Disease (2020)
-
Oncolytic Viruses for the Treatment of Metastatic Melanoma
Current Treatment Options in Oncology (2020)
-
Bi- and tri-valent T cell engagers deplete tumour-associated macrophages in cancer patient samples
Journal for ImmunoTherapy of Cancer (2019)
-
Combining Tumor Vaccination and Oncolytic Viral Approaches with Checkpoint Inhibitors: Rationale, Pre-Clinical Experience, and Current Clinical Trials in Malignant Melanoma
American Journal of Clinical Dermatology (2018)