Abstract
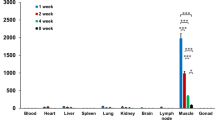
Preclinical biodistribution studies with INGN 007, an oncolytic adenovirus (Ad) vector, supporting an early stage clinical trial were conducted in Syrian hamsters, which are permissive for Ad replication, and mice, which are a standard model for assessing toxicity and biodistribution of replication-defective (RD) Ad vectors. Vector dissemination and pharmacokinetics following intravenous administration were examined by real-time PCR in nine tissues and blood at five time points spanning 1 year. Select organs were also examined for the presence of infectious vector/virus. INGN 007 (VRX-007), wild-type Ad5 and AdCMVpA (an RD vector) were compared in the hamster model, whereas only INGN 007 was examined in mice. DNA of all vectors was widely disseminated early after injection, but decayed rapidly in most organs. In the hamster model, DNA of INGN 007 and Ad5 was more abundant than that of the RD vector AdCMVpA at early times after injection, but similar levels were seen later. An increased level of INGN 007 and Ad5 DNA but not AdCMVpA DNA in certain organs early after injection, and the presence of infectious INGN 007 and Ad5 in lung and liver samples at early times after injection, strongly suggests that replication of INGN 007 and Ad5 occurred in several Syrian hamster organs. There was no evidence of INGN 007 replication in mice. In addition to providing important information about INGN 007, the results underscore the utility of the Syrian hamster as a permissive immunocompetent model for Ad5 pathogenesis and oncolytic Ad vectors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Everts B, van der Poel H . Replication-selective oncolytic viruses in the treatment of cancer. Cancer Gene Ther 2005; 12: 141–161.
Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science 1996; 274: 373–376.
Lichtenstein DL, Wold WSM . Experimental infections of humans with wild-type adenoviruses and with replication-competent adenovirus vectors: replication, safety, and transmission. Cancer Gene Ther 2004; 11: 819–829.
Wold WSM, Cladaras C, Magie SC, Yacoub N . Mapping a new gene that encodes an 11 600-molecular-weight protein in the E3 transcription unit of adenovirus 2. J Virol 1984; 52: 307–313.
Tollefson AE, Ryerse JS, Scaria A, Hermiston TW, Wold WSM . The E3-11.6 kDa adenovirus death protein (ADP) is required for efficient cell death: characterization of cells infected with adp mutants. Virology 1996; 220: 152–162.
Tollefson AE, Scaria A, Hermiston TW, Ryerse JS, Wold LJ, Wold WSM . The adenovirus death protein (E3-11.6K) is required at very late stages of infection for efficient cell lysis and release of adenovirus from infected cells. J Virol 1996; 70: 2296–2306.
Tollefson AE, Scaria A, Ying B, Wold WSM . Mutations within the ADP (E3-11.6K) protein alter processing and localization of ADP and the kinetics of cell lysis of adenovirus infected cells. J Virol 2003; 77: 7764–7778.
Ying B, Wold WSM . Adenovirus ADP protein (E3-11.6K), which is required for efficient cell lysis and virus release, interacts with human MAD2B. Virology 2003; 313: 224–234.
Doronin K, Toth K, Kuppuswamy M, Ward P, Tollefson AE, Wold WSM . Tumor-specific, replication-competent adenovirus vectors overexpressing the adenovirus death protein. J Virol 2000; 74: 6147–6155.
Doronin K, Kuppuswamy M, Toth K, Tollefson AE, Krajcsi P, Krougliak V et al. Tissue-specific, tumor-selective, replication-competent adenovirus vector for cancer gene therapy. J Virol 2001; 75: 3314–3324.
Doronin K, Toth K, Kuppuswamy M, Krajcsi P, Tollefson AE, Wold WSM . Overexpression of the ADP (E3-11.6K) protein increases cell lysis and spread of adenovirus. Virology 2003; 305: 378–387.
Habib NA, Mitry R, Seth P, Kuppuswamy M, Doronin K, Toth K et al. Adenovirus replication-competent vectors (KD1, KD3) complement the cytotoxicity and transgene expression from replication-defective vectors (Ad-GFP, Ad-Luc). Cancer Gene Ther 2002; 9: 651–654.
Kuppuswamy M, Spencer JF, Doronin K, Tollefson AE, Wold WS, Toth K . Oncolytic adenovirus that overproduces ADP and replicates selectively in tumors due to hTERT promoter-regulated E4 gene expression. Gene Therapy 2005; 12: 1608–1617.
Toth K, Tarakanova V, Doronin K, Ward P, Kuppuswamy M, Locke JL et al. Radiation increases the activity of oncolytic adenovirus cancer gene therapy vectors that overexpress the ADP (E3-11.6K) protein. Cancer Gene Ther 2003; 10: 193–200.
Toth K, Djeha H, Ying BL, Tollefson AE, Kuppuswamy M, Doronin K et al. An oncolytic adenovirus vector combining enhanced cell-to-cell spreading, mediated by the ADP cytolytic protein, with selective replication in cancer cells with deregulated Wnt signaling. Cancer Res 2004; 64: 3638–3644.
Barton KN, Paielli D, Zhang Y, Koul S, Brown SL, Lu M et al. Second-generation replication-competent oncolytic adenovirus armed with improved suicide genes and ADP gene demonstrates greater efficacy without increased toxicity. Mol Ther 2006; 13: 347–356.
Bauzon M, Castro D, Karr M, Hawkins LK, Hermiston TW . Regulated, multi-gene expression from a replicating adenovirus using native viral promoters. Mol Ther 2003; 7: 526–534.
Kohno S, Nakagawa K, Hamada K, Harada H, Yamasaki K, Hashimoto K et al. Midkine promoter-based conditionally replicative adenovirus for malignant glioma therapy. Oncol Rep 2004; 12: 73–78.
Oosterhoff D, Pinedo HM, Witlox MA, Carette JE, Gerritsen WR, Van Beusechem VW . Gene-directed enzyme prodrug therapy with carboxylesterase enhances the anticancer efficacy of the conditionally replicating adenovirus AdDelta24. Gene Therapy 2005; 12: 1011–1018.
Ramachandra M, Rahman A, Zou A, Vaillancourt M, Howe JA, Antelman D et al. Re-engineering adenovirus regulatory pathways to enhance oncolytic specificity and efficacy. Nat Biotechnol 2001; 19: 1035–1041.
Suzuki K, Alemany R, Yamamoto M, Curiel DT . The presence of the adenovirus E3 region improves the oncolytic potency of conditionally replicative adenoviruses. Clin Cancer Res 2002; 8: 3348–3359.
Yu DC, Chen Y, Seng M, Dilley J, Henderson DR . The addition of adenovirus type 5 region E3 enables calydon virus 787 to eliminate distant prostate tumor xenografts. Cancer Res 1999; 59: 4200–4203.
Zhu M, Bristol JA, Yuefong X, Mina M, Ji H, Forry-Schaudies S et al. Linked tumor-selective virus replication and transgene expression from E3-containing oncolytic adenoviruses. J Virol 2005; 79: 5455–5465.
Bernt KM, Ni S, Gaggar A, Li ZY, Shayakhmetov DM, Lieber A . The effect of sequestration by nontarget tissues on anti-tumor efficacy of systemically applied, conditionally replicating adenovirus vectors. Mol Ther 2003; 8: 746–755.
Huang D, Pereboev AV, Korokhov N, He R, Larocque L, Gravel C et al. Significant alterations of biodistribution and immune responses in Balb/c mice administered with adenovirus targeted to CD40(+) cells. Gene Therapy 2008; 15: 298–308.
Huang X, Zhuang L, Cao Y, Gao Q, Han Z, Tang D et al. Biodistribution and kinetics of the novel selective oncolytic adenovirus M1 after systemic administration. Mol Cancer Ther 2008; 7: 1624–1632.
Kanerva A, Wang M, Bauerschmitz GJ, Lam JT, Desmond RA, Bhoola SM et al. Gene transfer to ovarian cancer versus normal tissues with fiber-modified adenoviruses. Mol Ther 2002; 5: 695–704.
Paielli DL, Wing MS, Rogulski KR, Gilbert JD, Kolozsvary A, Kim JH et al. Evaluation of the biodistribution, persistence, toxicity, and potential of germ-line transmission of a replication-competent human adenovirus following intraprostatic administration in the mouse. Mol Ther 2000; 1: 263–274.
Stone D, Liu Y, Li ZY, Tuve S, Strauss R, Lieber A . Comparison of adenoviruses from species B, C, E, and F after intravenous delivery. Mol Ther 2007; 15: 2146–2153.
Duncan SJ, Gordon FC, Gregory DW, McPhie JL, Postlethwaite R, White R et al. Infection of mouse liver by human adenovirus type 5. J Gen Virol 1978; 40: 45–61.
Ginsberg HS, Moldawer LL, Sehgal PB, Redington M, Kilian PL, Chanock RM et al. A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc Natl Acad Sci USA 1991; 88: 1651–1655.
Hjorth RN, Bonde GM, Pierzchala WA, Vernon SK, Wiener FP, Levner MH et al. A new hamster model for adenoviral vaccination. Arch Virol 1988; 100: 279–283.
Liu TC, Hallden G, Wang Y, Brooks G, Francis J, Lemoine N et al. An E1B-19 kDa gene deletion mutant adenovirus demonstrates tumor necrosis factor-enhanced cancer selectivity and enhanced oncolytic potency. Mol Ther 2004; 9: 786–803.
Oualikene W, Gonin P, Eloit M . Short and long term dissemination of deletion mutants of adenovirus in permissive (cotton rat) and non-permissive (mouse) species. J Gen Virol 1994; 75: 2765–2768.
Lubeck MD, Davis AR, Chengalvala M, Natuk RJ, Morin JE, Molnar-Kimber K et al. Immunogenicity and efficacy testing in chimpanzees of an oral hepatitis B vaccine based on live recombinant adenovirus. Proc Natl Acad Sci USA 1989; 86: 6763–6767.
Lubeck MD, Natuk R, Myagkikh M, Kalyan N, Aldrich K, Sinangil F et al. Long-term protection of chimpanzees against high-dose HIV-1 challenge induced by immunization. Nat Med 1997; 3: 651–658.
Ni S, Bernt K, Gaggar A, Li ZY, Kiem HP, Lieber A . Evaluation of biodistribution and safety of adenovirus vectors containing group B fibers after intravenous injection into baboons. Hum Gene Ther 2005; 16: 664–677.
Pacini DL, Dubovi EJ, Clyde Jr WA . A new animal model for human respiratory tract disease due to adenovirus. J Infect Dis 1984; 150: 92–97.
Page JG, Tian B, Schweikart K, Tomaszewski J, Harris R, Broadt T et al. Identifying the safety profile of a novel infectivity-enhanced conditionally replicative adenovirus, Ad5-delta24-RGD, in anticipation of a phase I trial for recurrent ovarian cancer. Am J Obstet Gynecol 2007; 196: 389.e1-9; discussion 389.e9-10.
Prince GA, Porter DD, Jenson AB, Horswood RL, Chanock RM, Ginsberg HS . Pathogenesis of adenovirus type 5 pneumonia in cotton rats (Sigmodon hispidus). J Virol 1993; 67: 101–111.
Torres JM, Alonso C, Ortega A, Mittal S, Graham F, Enjuanes L . Tropism of human adenovirus type 5-based vectors in swine and their ability to protect against transmissible gastroenteritis coronavirus. J Virol 1996; 70: 3770–3780.
Toth K, Spencer JF, Tollefson AE, Kuppuswamy M, Doronin K, Lichtenstein DL et al. Cotton rat tumor model for the evaluation of oncolytic adenoviruses. Hum Gene Ther 2005; 16: 139–146.
Wildner O, Morris JC . Subcutaneous administration of a replication-competent adenovirus expressing HSV-tk to cotton rats: dissemination, persistence, shedding, and pathogenicity. Hum Gene Ther 2002; 13: 101–112.
Thomas MA, Spencer JF, La Regina MC, Dhar D, Tollefson AE, Toth K et al. Syrian hamster as a permissive immunocompetent animal model for the study of oncolytic adenovirus vectors. Cancer Res 2006; 66: 1270–1276.
Thomas MA, Spencer JF, Toth K, Sagartz JE, Phillips NJ, Wold WSM . Immunosuppression leads to increased oncolytic adenovirus efficacy and regression of large tumors in the Syrian hamster model. Mol Ther 2008; 16: 1665–1673.
Toth K, Spencer JF, Dhar D, Sagartz JE, Buller RM, Painter GR et al. Hexadecyloxypropyl-cidofovir, CMX001, prevents adenovirus-induced mortality in a permissive, immunosuppressed animal model. Proc Natl Acad Sci USA 2008; 105: 7293–7297.
Lichtenstein DL, Spencer JF, Patra D, Meyer J, Shashkova EV, Kuppuswamy M et al. An acute toxicology study with INGN 007, an oncolytic adenovirus vector, in permissive Syrian hamsters and nonpermissive mice; comparisons with wild-type Ad5 and a replication-defective vector. Cancer Gene Ther 2008 In press.
Shayakhmetov DM, Li ZY, Ni S, Lieber A . Analysis of adenovirus sequestration in the liver, transduction of hepatic cells, and innate toxicity after injection of fiber-modified vectors. J Virol 2004; 78: 5368–5381.
Shayakhmetov DM, Gaggar A, Ni S, Li ZY, Lieber A . Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J Virol 2005; 79: 7478–7491.
Wood M, Perrotte P, Onishi E, Harper ME, Dinney C, Pagliaro L et al. Biodistribution of an adenoviral vector carrying the luciferase reporter gene following intravesical or intravenous administration to a mouse. Cancer Gene Ther 1999; 6: 367–372.
Worgall S, Wolff G, Falck-Pedersen E, Crystal RG . Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum Gene Ther 1997; 8: 37–44.
Hallden G, Hill R, Wang Y, Anand A, Liu TC, Lemoine NR et al. Novel immunocompetent murine tumor models for the assessment of replication-competent oncolytic adenovirus efficacy. Mol Ther 2003; 8: 412–424.
Baker AH, Mcvey JH, Waddington SN, Di Paolo NC, Shayakhmetov DM . The influence of blood on in vivo adenovirus bio-distribution and transduction. Mol Ther 2007; 15: 1410–1416.
Acknowledgements
This research was supported by grants CA118022, CA108335 and CA81829 from the National Institutes of Health to WSMW. Funding for this work was also supported by a research and development agreement to VirRx Inc. from Introgen Therapeutics Inc.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure/Conflict of interest
WSMW, KT, AET, KD, MK and Introgen Therapeutics Inc. own shares of VirRx Inc.
Rights and permissions
About this article
Cite this article
Ying, B., Toth, K., Spencer, J. et al. INGN 007, an oncolytic adenovirus vector, replicates in Syrian hamsters but not mice: comparison of biodistribution studies. Cancer Gene Ther 16, 625–637 (2009). https://doi.org/10.1038/cgt.2009.6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2009.6
Keywords
This article is cited by
-
A novel immunocompetent murine model for replicating oncolytic adenoviral therapy
Cancer Gene Therapy (2015)
-
The role of cyclophosphamide in enhancing antitumor efficacy of an adenovirus oncolytic vector in subcutaneous Syrian hamster tumors
Cancer Gene Therapy (2013)
-
Mining the adenovirus virome for oncolytics against multiple solid tumor types
Cancer Gene Therapy (2011)
-
Current Issues and Future Directions of Oncolytic Adenoviruses
Molecular Therapy (2010)
-
A fully replication-competent adenovirus vector with enhanced oncolytic properties
Cancer Gene Therapy (2010)