Abstract
On the basis of phylogenetic analyses, we uncovered a variant of the CDX2 homeobox gene, a major regulator of the development and homeostasis of the gut epithelium, also involved in cancer. This variant, miniCDX2, is generated by alternative splicing coupled to alternative translation initiation, and contains the DNA-binding homeodomain but is devoid of transactivation domain. It is predominantly expressed in crypt cells, whereas the CDX2 protein is present in crypt cells but also in differentiated villous cells. Functional studies revealed a dominant-negative effect exerted by miniCDX2 on the transcriptional activity of CDX2, and conversely similar effects regarding several transcription-independent functions of CDX2. In addition, a regulatory role played by the CDX2 and miniCDX2 homeoproteins on their pre-mRNA splicing is displayed, through interactions with splicing factors. Overexpression of miniCDX2 in the duodenal Brunner glands leads to the expansion of the territory of these glands and ultimately to brunneroma. As a whole, this study characterized a new and original variant of the CDX2 homeobox gene. The production of this variant represents not only a novel level of regulation of this gene, but also a novel way to fine-tune its biological activity through the versatile functions exerted by the truncated variant compared to the full-length homeoprotein. This study highlights the relevance of generating protein diversity through alternative splicing in the gut and its diseases.
Similar content being viewed by others
Main
The intestinal epithelium is a complex cellular system in constant renewal whose dynamic homeostasis involves multiple and complementary factors and pathways.1, 2 Among them, the homeotic transcription factor CDX2 has a central role during embryogenesis to determine the intestinal identity of the presumptive midgut/hindgut endoderm,3, 4 and throughout adulthood for intestinal epithelium homeostasis to organize the stem cell niche, to provide tissue identity to the stem cells, and to regulate cell proliferation and differentiation.5, 6, 7, 8 CDX2 gene expression and function need to be tightly regulated, because both loss and overexpression are lethal.8, 9 CDX2 is also relevant in gut pathologies, since its strong decay in human colon cancer correlates with poor evolution,10, 11 while functional studies have attributed a tumor suppressor role in the gut.12, 13, 14, 15
Alternative splicing of pre-messenger RNA is an important facet of RNA metabolism to generate protein diversity16, 17 involved in every biological process from embryonic development to tissue homeostasis, and also in diseases.18, 19 However, it remains largely underrated because most of the splicing variants are only identified by their sequence without knowledge on their function. Alternative splicing is poorly documented in the gut. Here, we discovered a splicing variant of CDX2 and we unveiled its original function.
Results
The CDX2 homeobox gene encodes an alternative mRNA variant, miniCDX2
To search for novel transcript isoforms in the mouse intestine, 490 million paired-end reads were generated from three independent RNA samples of the cecum. They corresponded to 16 822 consistently expressed genes (>10 normalized reads in all replicates), and represented 43% of all annotated genes in the mouse genome including, for instance, 98% of the intestinal stem cells markers.20 The comparison to the reference transcripts annotation identified 4799 novel possible exon–exon junctions in 1802 genes (Supplementary Table 1). This highlights the potential of alternative splicing to generate protein diversity in the gut.
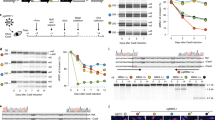
Among those genes is CDX2, a homeobox gene containing three exons (Figure 1A). Exploring its functional structure by RT-PCR and northern blot in human colon cancer cell lines and mouse intestine identified the variant referred to as miniCDX2 (Figure 1B). The level of this variant was far below that of the CDX2 mRNA (15-times less abundant) in proliferating Caco2TC7 cells (3 days in culture) and further decreased in differentiated cells (14 days in culture). The miniCDX2 mRNA was up to 20-times less abundant than the CDX2 mRNA along the murine intestine.
Alternative splicing at the CDX2 locus. (A) CDX2 gene map. E1–3: exon-1 to -3; I1 and 2: intron-1 and -2; dotted lines represent the spliced regions to produce, respectively, the CDX2 and miniCDX2 mRNAs. The translation start codons ATG1 and ATG2, and the stop codons Stop1 and Stop2 are indicated. (B) Expression of the miniCDX2 transcript. (a) RT-PCR on intestinal cell lines with the primers CDX21F/CCR hybridizing, respectively, upstream of ATG1 in the exon-1 and downstream of Stop2 in the exon-3. The two bands correspond to the CDX2 and miniCDX2 transcripts. (b) Northern blot of polyA RNA (10 μg per lane) from Caco2TC7 cells cultured for 3 days and 14 days revealing the CDX2 and miniCDX2 mRNAs. (c) RT-qPCR quantification of CDX2 (light gray) and miniCDX2 mRNA (dark gray) in 3 days Caco2TC7 cells (semi-logarithmic scale). (d) RT-qPCR of CDX2 (light gray) and miniCDX2 mRNA (dark gray) along the murine gut (semi-logarithmic scale); CDX2 was arbitrary set at 1 in the cecum. (e) CDX2 / miniCDX2 mRNA ratio along the mouse intestine
MiniCX2 corresponds to an evolutionary-conserved alternative splicing variant
The production of the miniCDX2 variant involves a canonical GU splicing donor site within the exon-1, instead of the standard donor site at the end of the exon used to produce the CDX2 mRNA (Supplementary Figure S1). Remarkably, the alternative donor site overlaps the translation initiation codon of the CDX2 protein in the CDX2 mRNA (referred thereafter to as AUG2) that is therefore destroyed in the miniCDX2 mRNA. Yet, the CDX2 gene sequence contains one additional AUG located 29 pb upstream of AUG2. This AUG, referred to as AUG1, is out-of-frame with AUG2 but in-frame with the open reading frame of exon-2 and -3 in the miniCDX2 transcript. AUG1 is preceded by GGCAGC and AUG2 by GCCACC, both being consistent with a consensus Kozak box (GNCANCAUG).21 The CDX2 mRNA, but not the miniCDX2 variant, additionally contains a translation stop codon (Stop1) located four nucleotides downstream of AUG2, thus in-frame with AUG1.
Strikingly, the motif composed of (i) the GU splicing donor site overlapping the AUG2 start codon of the CDX2 protein, (ii) the putative upstream start site AUG1 and (iii) the stop codon Stop1 in-frame with AUG1, is evolutionary-conserved in vertebrates from coelacanth to human, including marsupials, birds, reptiles, amphibians, the bichir (the most basal extant ray-finned fish) and the bowfin (the immediate outgroup to teleost fish) (Figure 2A; Supplementary Figure S2). However, it is found neither in CDX1-type genes (except for Cdx1b in teleost fishes like Danio rerio, see ‘Discussion’), nor in CDX4-type genes.
Functionality of the conserved alternative splicing/translation motif of the CDX2 locus. (A) The conserved motif found in CDX2-type genes (see also the Supplementary Figure 2). (B) (a) Luciferase activity in human colon cancer HCT116 cells transfected with the indicated reporter plasmids; X designates the mutation of ATG1 into TAG in pATG1m and in pATG1m-ATG2; pGl3-control was set to 100 and pGl3-basic gave background activity. (b) Luciferase activity in HCT116 cells transfected with in vitro-transcribed polyadenylated RNA without (light gray) or with m7G cap (dark gray). Data are given as mean±S.D. for triplicates
The miniCDX2 variant encodes a truncated form of the CDX2 homeoprotein
To study the translational activity of the upstream AUG1 and to compare it with AUG2 used for CDX2 translation, reporter plasmids were constructed in which the 5′-untranslated sequence of the CDX2 gene ending at ATG2 (pATG1-ATG2) or ATG1 (pATG1) was linked to the Luciferase coding sequence (Figure 2Ba). The translation activity of AUG2 used for CDX2 synthesis was verified by the production of Luciferase in HCT116 cells transfected with pATG1-ATG2 that recapitulates the configuration of the CDX2 mRNA in which AUG2 is in-frame and AUG1 out-of-frame with the reporter sequence. Interestingly, pATG1 ending at ATG1 and recapitulating the miniCDX2 configuration produced an even higher luciferase activity than pATG1-ATG2. This activity was abolished by mutating ATG1 into TAG in pATG1m, while changing ATG1 into TAG in pATG1m-ATG2 increased luciferase compared to pATG1-ATG2. Thus, AUG1 is translationally active and, in the context of the CDX2 mRNA, it partially hinders the translational activity of AUG2.
In the CDX2 mRNA, the AUG2 start codon used for the CDX2 protein is preceded by the translationally competent AUG1. Ribosomes generally start translation at the first AUG after cap-dependent scanning of the 5′-untranslated region, but can skip it for a second site by leaky scanning22 or reach an internal codon using a cap-independent internal ribosome entry site.23 To compare the cap-dependency of AUG1 and AUG2, uncapped and m7G-capped reporter RNAs named RNAAUG1 and RNAAUG1-AUG2 were in vitro transcribed from PCR templates derived from pATG1 and pATG1-ATG2, respectively, and transfected in HCT116 cells. Luciferase activity was higher for capped versus uncapped RNAAUG1, and for capped versus uncapped RNAAUG1-AUG2. Moreover, destroying AUG1 in RNAAUG1m-AUG2 increased luciferase compared to RNAAUG1-AUG2 (Figure 2Bb). Thus, the activity of AUG2, like AUG1, is cap-dependent, suggesting that ribosomes reach AUG2 for CDX2 translation by leaky scanning through AUG1.
The sequence of the splicing mRNA variant predicts the production of a CDX2-related homeoprotein, miniCDX2, in which a 12-aa N-terminus segment replaces the 181-aa transactivation domain (Figure 3A, Supplementary Figure S1). An antibody, C2T, was raised against this 12-aa segment and validated using expression plasmids (Figure 3Ba), peptide competition and conditional CDX2 knockout mice (Figure 3Bc). Consistent with the presence of the DNA-binding homeodomain, the miniCDX2 protein produced by pFlag-miniCDX2-transfected HCT116 cells was nuclear (Figure 3Bb). Compared to CDX2 present in almost every intestinal crypt and villous epithelial cell from embryos to adults, miniCDX2 was not detected in the embryonic gut, it appeared perinatally at the level of the intervillous epithelium at the origin of the crypts (Figure 3Ca), and remained expressed throughout adult life in the small intestinal crypts and in the bottom of the colonic glands (Figures 3Ca and c). Crypt cells co-expressed miniCDX2 and CDX2 (Figure 3Cb).
Protein expression of the miniCDX2 variant. (A) Structure of the CDX2 and miniCDX2 proteins: TA, transactivation domain; HD, DNA-binding homeodomain; R, regulatory domain. The arrow points to the 12-aa Nter peptide of miniCDX2. (B) The C2T antibody raised against the 12-aa Nter peptide of miniCDX2. (a) HCT116 cells transfected with the plasmid pFlag-CDX2 (lane 1), pFlag-miniCDX2 (lane 2) or the empty vector pFlag-CMV2 (lane 3) were analyzed by western blot with Flag, CDX2 or C2T antibodies. The Flag antibody reveals both Flag-CDX2 and Flag-miniCDX2 proteins, whereas the CDX2 and C2T antibodies, respectively, detect Flag-CDX2 and Flag-miniCDX2. (b) HCT116 cells co-transfected with pFlag-miniCDX2 and pEGFP showed nuclear localization of Flag-miniCDX2 (Flag antibody, red). Bars: 10 μm. (c) Validation of the C2T antibody in mouse intestinal sections: nuclear immunostaining in the crypt epithelium of wild-type mice; loss of staining when C2T is preincubated with an excess of immunogenic peptide; absence of immunostaining in the intestinal epithelium of conditional knockout (KO) Cdx2f/f::AhCreERT mice.7 Bars: 50 μm. (C) Immunostaining pattern of the miniCDX2 (C2T antibody) and CDX2 proteins in the small intestine of E15 mouse embryos, and of post-natal day 2 (P2) and day 11 (P11) suckling and adult (Ad) mice. (b) Co-immunofluorescence detection of the miniCDX2 (C2T) and CDX2 proteins in the small intestine of adult mice. Bars: 100 μm. (c) Immunostaining of the miniCDX2 (C2T) and CDX2 proteins in the proximal colon of adult mice. Bars: 50 μm. Higher magnification pictures are given in the Supplementary Figure S3A. (D) MiniCDX2 expression in tumors of the proximal ileum of ApcΔ14/+ mice. (a) HE staining and C2T and CDX2 immunostaining. The region boxed in HE is shown in the immunohistochemistry pictures. Bars: 100 μm. (b) RT-qPCR quantification of CDX2 (light gray) and miniCDX2 mRNA (dark gray) (semi-logarithmic scale) in wild-type ileum (wt), in three ileal tumors of ApcΔ14/+ mice (Apc T1-3) and in the adjacent normal mucosa (Apc N1-3); data are related to the amount of CDX2 mRNA arbitrary set at 1 in the wild-type ileum. (c) Ratio between the CDX2 and miniCDX2 transcripts
In small intestinal tumors of ApcΔ14/+ mice, CDX2 expression is known to become reduced and heterogeneous. Interestingly, miniCDX2 was also expressed heterogeneously and in the same tumor regions as CDX2 (Figure 3Da). Moreover, RT-qPCR showed that the ratio between the CDX2 and miniCDX2 transcripts decreased in these tumors compared to the normal mucosa (P<0.05) (Figures 3Db and c).
Opposite and similar molecular functions of miniCDX2 compared to CDX2
We next addressed the molecular functions of miniCDX2 compared to CDX2. As a transcription factor, the CDX2 protein activates a large number of downstream genes including the sucrase–isomaltase (SI) gene. In HCT116 cells transfected with pFlag-CDX2 or pFlag-miniCDX2, the CDX2 protein, but not miniCDX2, stimulated the level of SI mRNA and the activity of the SI gene promoter assayed using the SI luciferase reporter; moreover, miniCDX2 dose-dependently abolished the stimulatory effect of CDX2 (Figures 4Aa and b). Chromatin immunoprecipitation (ChIP) revealed the ability of miniCDX2 to bind the same chromatin targets as CDX2, for example, the SI, Li-cadherin and Muc2 promoters (Figure 4Ac), and to reduce the binding of CDX2 to the SI promoter (Figure 4Ad). Thus, miniCDX2 is transcriptionally inefficient, but acts as a dominant-negative regulator of the transcriptional activity of CDX2 by DNA-binding competition.
Molecular functions of miniCDX2 compared to CDX2. (A) Effect of miniCDX2 on the transcriptional activity of the CDX2 protein. (a) RT-qPCR quantification of the SI RNA and (b) pSI-luc luciferase activity in HCT116 cells transfected either with pFlag-miniCDX2 or pFlag-CDX2 or pFlag-CDX2 together with increasing amounts of pFlag-miniCDX2; data are given as mean±S.D. for triplicates (***P<0.001). (c) ChIP with anti-Flag or IgG in HCT116 cells transfected with pFlag-CMV2 (lane 1), pFlag-CDX2 (lane 2) or pFlag-miniCDX2 (lane 3); co-precipitated DNA was PCR-amplified with primers of the SI, LI-cadherin and Muc2 promoters. (d) ChIP with anti-CDX2 or IgG in HCT116 cells transfected either with pFlag-CMV2 (lane 1) or pFlag-CDX2 (lane 2) or pFlag-CDX2 together with a threefold molar excess of pFlag-miniCDX2 (lane 3) or pFlag-miniCDX2 (lane 4); co-precipitated DNA was PCR-amplified with primers of the SI promoter. (B) Effect on miniCDX2 on β-catenin/Tcf4 signaling. (a) RT-qPCR quantification of the Mmp7 RNA and (b) TOP-Flash luciferase activity in HEK293 cells transfected with pS33A-β-catenin, pMyc-Tcf4, pFlag-CDX2 and/or pFlag-miniCDX2; data are given as mean±S.D. for triplicates (***P<0.001). (c) Proteins from cells transfected either with pS33A-β-catenin alone (lane 1), or pS33A-β-catenin together with pFlag-CDX2 (lane 2), or pS33A-β-catenin together with pFlag-miniCDX2 (lane 3) were immunoprecipitated with anti-β-catenin or IgG, and analyzed by western blot with anti-β-catenin and with anti-Flag to reveal Flag-CDX2 (⊳) and Flag-miniCDX2 (▸). (d) Proteins from cells transfected with pS33A-β-catenin and pMyc-Tcf4 together with increasing amounts of pFlag-CDX2 or pFlag-miniCDX2 were immunoprecipitated with anti-β-catenin, and analyzed by western blot with anti-β-catenin and anti-Myc to check the β-catenin/Tcf4 interaction. (C) Effect of miniCDX2 on P27KIP1. (a) Representative western blot and quantification and (b) RT-qPCR quantification of P27KIP1 protein and RNA in HCT116 cells transfected either with pFlag-CMV2 (ctrl) or pFlag-CDX2 or pFlag-miniCDX2. (c) Representative pictures and quantification of clonogenic assays on HCT116 cells transfected either with pFlag-CMV2 (ctrl) or pFlag-CDX2 or pFlag-miniCDX2. Data are means±S.D. for triplicates (**P<0.01). (D) Effect of miniCDX2 on double-strand break DNA repair. (a) Proteins from cells transfected either with pFlag-CMV2 (lane 1), or pFlag-CDX2 (lane 2) or pFlag-miniCDX2 (lane 3) were immunoprecipitated with anti-Flag or IgG, and analyzed by western blot with anti-Flag and anti-KU70/80 to check the interaction of Flag-CDX2 (⊳) or Flag-miniCDX2 (▸) with the KU70/80 complex. (b) For DNA repair assays, linearized plasmid pcDNA3 (lane 1) was incubated with nuclear extracts of SW480 cells transfected with pFlag-CMV2 (lane 2), pFlag-CDX2 (lane 3) or pFlag-miniCDX2 (lane 4), and then separated by electrophoresis on an agarose gel. The linear and repaired (circular and/or concatemers) plasmid forms are indicated
CDX2 exerts broader functions than regulating transcription through DNA binding.24 For instance, it can bind β-catenin to prevent its interaction with Tcf4, thus impeding Wnt/β-catenin signaling.25 Indeed, CDX2 opposed the stimulation of the Mmp7 mRNA, a Wnt target, and the activity of the TOP-Flash Wnt reporter by β-catenin/Tcf4 (Figures 4Ba and b). Unlike CDX2, miniCDX2 failed to interfere with the β-catenin/Tcf4 stimulation of Mmp7 mRNA and TOP-Flash activity. In addition, it failed to relieve the inhibitory effect of CDX2 on β-catenin/Tcf4 (Figures 4Ba and b), and did neither interact with β-catenin nor prevent β-catenin/Tcf4 interaction (Figures 4Bc and d). Thus, contrary to its dominant-negative effect on the CDX2 transcriptional activity, miniCDX2 is neutral with respect to the inhibitory function exerted by CDX2 on β-catenin/Tcf4 signaling.
CDX2 also exerts transcription-independent functions including the increase of P27KIP1 to compromise cell proliferation.26 Like CDX2, miniCDX2 increased the level of P27KIP1 protein without changing its mRNA (Figures 4Ca and b) and it reduced cell growth in a colony formation assay (Figure 4Cc). The CDX2 protein has also been reported to inhibit double-stranded DNA breaks repair in HCT116 cells by interacting with the KU70/80 complex.27 Here, we extended these observations by showing in SW480 colon cancer cells that miniCDX2, like CDX2, also co-immunoprecipitated with KU70/80 proteins, and hampered DNA repair activity (Figures 4Da and b).
Thus, miniCDX2 fine-tunes the activity of CDX2 in that it exerts similar and/or different effects with respect to the multifaceted functions of the full-length homeoprotein: it is dominant-negative on the transcriptional activity of CDX2, it is neutral as regards the inhibitory role of CDX2 on β-catenin/Tcf4 interaction, and it exerts similar transcription-independent effects as CDX2 on cell growth inhibition by P27KIP1 and on double-stranded DNA break repair through KU70/80.
MiniCDX2 participates in the control of intestinal homeostasis
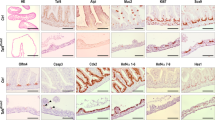
We next explored the role of miniCDX2 in vivo in mice using a gain-of-function approach. For this purpose, transgenic jojo-Flag-miniCDX2 mice were generated, allowing inducible expression of the Flag-miniCDX2 protein (Figure 5A). Crossing jojo-Flag-miniCDX2 and Sox2Cre mice to broadly express Flag-miniCDX2 in the zygote caused embryonic death, which is reminiscent of the lethality resulting from CDX2 knockout (0 living double-transgenics over 80 descendants). In the adult gut of jojo-Flag-miniCDX2 mice (three independent founders), GFP immunostaining used to trace the activity of the CAG promoter revealed only very few villi with transgene expression (<1%, Supplementary Figure S3Ba). Consequently, jojo-Flag-miniCDX2::VilCre mice generated to target the intestinal epithelium showed only rare villi with Flag-miniCDX2 expression. In those villi with a sustained expression of Flag-miniCDX2, intestinal alkaline phosphatase was lost, whereas gastric Claudin-18 was turned on (Figure 5B), without apparent change in CDX2. This reproduced the differentiation switch induced by targeted CDX2 knockout in the gut,28 indicating that miniCDX2 is able to antagonize the transcriptional potential of CDX2 in vivo.
Overexpression of miniCDX2 in transgenic mice. (A) Map of the jojo-Flag-miniCDX2 transgene with the CAG promoter, the loxP/Cre-excisable cassette containing the GFP coding sequence followed by a transcriptional stop (pA), and the Flag-miniCDX2 coding sequence. (B) Serial sections of rare small intestinal villi of jojo-Flag-miniCDX2::VilCre mice showing Flag-miniCDX2 expression accompanied by the loss of alkaline phosphatase and the ectopic expression of Claudin-18. Bars: 50 μm. (C) (a) Histology of the Brunner’s glands of jojo-Flag-miniCDX2::VilCre mice (miniCdx2) compared to control jojo-Flag-miniCDX2 animals (ctrl); bars: 500 μm. (b) Quantification of the surface of the Brunner’s glands in 3–4-month-old control jojo-Flag-miniCDX2 (ctrl, n=12) and jojo-Flag-miniCDX2::VilCre mice (miniCdx2, n=9); **P<0.01. (D) Pdx1 immunofluorescence staining in control jojo-Flag-miniCDX2 (ctrl) and jojo-Flag-miniCDX2::VilCre (miniCdx2) mice; bars: 100 μm. (E) The Brunner’s glands of the jojo-Flag-miniCDX2::VilCre mice show no major change in cell differentiation (PAS staining) and no obvious activation of the cell proliferation (P-Hist3). Bars: 100 μm. The asterisks label the duodenal crypts overlaying the Brunner’s glands
Brunner’s glands (BGs) are glandular structures connected to the crypts of the floor of the proximal duodenum. They express both CDX2 and miniCDX2 at low levels (not shown). Interestingly, the CAG promoter is active in the BGs of jojo-Flag-miniCDX2 mice, as revealed by GFP immunostaining, thus allowing a widespread expression of Flag-miniCDX2 in the BGs of jojo-Flag-miniCDX2::VilCre mice (Supplementary Figures S3Bb and C). Noteworthy, these mice exhibited a 2.5-fold enlargement of the territory of the BGs (Figure 5C). This was accompanied by a higher expression of PDX1, an important regulator of BG morphogenesis29 (Figure 5D) without change in cell proliferation and differentiation, as assessed by the absence of significant phospho-histone-3 labeling, and by the staining of neutral mucins (Figure 5E).
To address the consequence of the overgrowth of the BGs, mice were left to age until 18–24 months (n=19). Fifty-two percent (10/19) of jojo-Flag-miniCDX2::VilCre mice developed one to two polyps in the pyloric region (Figure 6A), in contrast to control jojo-Flag-miniCDX2 animals (1/12). These polyps exhibited low- to high-grade dysplasia, without sign of neoplasia. As illustrated in Figures 6A and B, they were formed by expanded BGs extending even under the gastric mucosa, covered by disorganized crypts from the overlying duodenal epithelium, and by a surface epithelium with foregut-type properties, exhibiting gastric Claudin-18 expression and occasionally P63-positive squamous stratified epithelium. Phospho-histone-3 proliferating cells concentrated in the disorganized crypt area. Consistent with the absence of neoplastic area, there was no evidence of β-catenin cytoplasmic/nuclear translocation. BGs produce EGF while adenomas develop after deletion of the EGF-receptor inhibitor Lrig1.30 RT-qPCR revealed a 4–18 times higher level of EGF mRNA in the polyps of jojo-Flag-miniCDX2::VilCre mice compared to the duodenal mucosa of control animals (n=3). Linked to that, the disorganized crypt area showed elevated levels of membranous phospho-EGF-receptor, as compared to normal adjacent duodenal crypts (Figure 6C). Thus, the miniCDX2-driven expansion of BGs leads to the overproduction of EGF that activates the EGFR and makes hyperproliferate the overlaying duodenal crypts, ultimately forming polyps.
Duodenal lesions in aged jojo-Flag-miniCDX2::VilCre mice. (A) (a) Macroscopic view of two adjacent polyps grown in the duodenal region of a 18-month-old jojo-Flag-miniCDX2::VilCre mouse. (b) Histology of a polyp near the gastric-intestinal boundary (arrow); (i), (ii) and (iii), respectively, designate the expanded territory of Brunner’s glands (encircled), the hyper-proliferating crypts and the surface area. The arrowhead locates squamous stratified epithelium. Bar: 500 μm. (B) Immunostaining of Flag-miniCDX2, phospho-histone-3, Claudin-18 and β-catenin in the regions (i), (ii) and (iii) of the duodenal polyp. Bars: 50 μm. (C) Phospho-EGFR immunodetection in the regions (i) and (ii) of the polyps, and in normal adjacent duodenal crypts. Bars: 50 μm
Autoregulation of the CDX2 pre-mRNA splicing
Cdx2+/− mice develop cecal heteroplasias exhibiting a shift toward a foregut-type differentiation.3 These lesions are devoid of CDX2 protein even though one gene copy is preserved. Since miniCDX2 overexpression also induces foregut-type transdifferentiation, this prompted us to look for miniCDX2 in the heteroplasias of Cdx2+/− mice. Unlike CDX2 present in the normal cecal epithelium but absent in the heteroplastic glands, miniCDX2 was detected not only in the normal epithelium but also in the heteroplastic structures, indicating a splicing shift in favor of miniCDX2 in the heteroplasias (Figure 7a). This opens the possibility that the balance between both splicing forms might be regulated and depend on the CDX2 protein itself.
Autoregulation of the CDX2 pre-mRNA splicing. (a) Immunodetection of the CDX2 and miniCDX2 (C2T antibody) proteins at the border between normal cecal epithelium and heteroplasia (asterisk) in Cdx2+/- mice. Bars: 50 μm. (b) Map of the eC2I1 reporter plasmid. (c) Production of the two protein variants CDX2-Flag (⊳) and miniCDX2-Flag (▸) from the reporter plasmid eC2I1. HCT116 cells were transfected either with the plasmids eC2I1, pFlag-CDX2 or pFlag-miniCDX2, and the proteins were revealed using anti-Flag, anti-CDX2 and anti-miniCDX2 (C2T) antibodies, respectively. (d) HCT116 cells transfected with the plasmid eC2I1 or with the mutant form eC2I1m. (e) Inverse correlation between the production of miniCDX2-Flag and the endogenous level of CDX2 in HCT116 and SW480 colon cancer cell lines. Upper panel: HCT116 and SW480 transfected with eC2I1. Lower panel: endogenous level of expression of CDX2 protein in HCT116 and SW480 cells related to actin. (f) HCT116 cells transfected with eC2I1 and either the control empty plasmid, or the plasmids encoding pHA-CDX2 or pHA-miniCDX2. The lower panel confirms the expression of HA-CDX2 and HA-miniCDX2 in these cells, using anti-HA antibody
To investigate the mechanism of pre-mRNA splicing, we constructed the plasmid eC2I1 overlapping the exon-1, intron-1 and the fused exon-2/-3 of the CDX2 gene, and having a Flag sequence preceding the Stop codon in exon-3 (Figure 7b). In HCT116 cells, eC2I1 encoded both CDX2-Flag and miniCDX2-Flag variants identified using Flag, CDX2 and C2T antibodies (Figure 7c). Mutating the GT alternative splicing donor site into GA to give eC2I1m compromised the production of miniCDX2-Flag (Figure 7d). Thus, eC2I1 represents an appropriate reporter system of the alternative splicing of the CDX2 pre-mRNA.
Interestingly, transfecting eC2I1 in HCT116 and SW480 cells produced different rates of miniCDX2-Flag protein, inversely related to the respective endogenous level of CDX2 in these cells (Figure 7e). To address a possible role of the CDX2 protein in regulating its pre-mRNA splicing, HCT116 cells were co-transfected with eC2I1 and the plasmid encoding HA-CDX2. HA-CDX2 reduced the amount of miniCDX2-Flag produced by eC2I1, and this effect was also recapitulated by the transcription-deficient protein HA-miniCDX2 (Figure 7f). Thus, the CDX2 and miniCDX2 proteins exert a similar inhibitory effect on the alternative splicing leading to the miniCDX2 variant, through a transcription-independent mechanism.
The sequence around the alternative splicing donor site of the CDX2 pre-mRNA revealed several putative splicing factors binding elements conserved in human and mouse (Supplementary Figure 4), in particular for the SRp30c and ASF/SF2 factors expressed in intestinal cell lines (Figure 8A). In transfected HCT116 cells, His-ASF/SF2 inhibited the splicing leading to miniCDX2-Flag, whereas His-SRp30c stimulated it; in addition, the stimulatory effect exerted by His-SRp30c was abolished by His-ASF/SF2 (Figure 8Ba). Of note, CDX2 also counteracted the effect of SRp30c (Figure 8Bb). Co-immunoprecipitation (coIP) was performed to investigate possible interactions between these factors. In transfected HCT116 cells, His-SRp30c co-immunoprecipitated with His-ASF/SF2 (Figure 8Ca). A similar result was obtained with the endogenous SRp30c and ASF/SF2 proteins, and the amount of co-precipitated material increased by RNase treatment, indicating an RNA-independent interaction (Figure 8Cb). Interestingly, HA-CDX2 also interacted with the endogenous ASF/SF2 (Figure 8Da) and SRp30c proteins (Figure 8Ea), and the amount of complex increased by RNase treatment. HA-miniCDX2 gave the same results, showing that the transactivation domain and activity of CDX2 is dispensable for its interaction with splicing factors (Figures 8Db). Hence, we investigated the impact of CDX2 and miniCDX2 on the ASF/SF2 / SRp30c complex. For this purpose, cells were transfected either with the control plasmid or pHA-CDX2 or pHA-miniCDX2, and nuclear extracts were processed for immunoprecipitation with anti-ASF/SF2 antibody. As shown in Figure 8F, the amount of SRp39c co-immunoprecipitated with ASF/SF2 increased in the presence of HA-CDX2 or HA-miniCDX2, respectively, by 1.7- and 1.6-fold compared to the control. Thus, CDX2 and miniCDX2 stimulate the SRp30c/ASF/SF2 interaction, which subsequently hampers the selection of the alternative splicing donor site used to produce the miniCDX2 splicing variant.
Mechanism of regulation of the CDX2 pre-mRNA splicing. (A) Expression of the splicing factors ASF/SF2 and SRp30c in HCT116 and SW480 colon cancer cells. (B) Impact of ASF/SF2, SRp30c and CDX2 on the reporter plasmid eC2I1. HCT116 cells were transfected with eC2I1 and the expression plasmids for the indicated proteins, and the two splicing variants CDX2-Flag (⊳) and miniCDX2-Flag (▸) were detected with anti-Flag antibody. (C) Interaction between ASF/SF2 and SRp30c. (a) HCT116 cells were transfected with the plasmids encoding His-ASF/SF2 and His-SRp30c. After immunoprecipitation with anti-ASF/SF2 antibody or with IgG, the proteins were revealed with anti-ASF/SF2 and anti-SRp30c antibodies. (b) Endogenous proteins of HCT116 cells were immunoprecipitated with IgG or with anti-ASF/SF2 antibody without or with pre-treatment with RNase, and revealed with anti-SRp30c antibody. (D) Interaction between ASF/SF2 and CDX2 or miniCX2. (a) HCT116 cells were transfected with the control plasmids pCMV2-Flag (lane 1) or pHA-CDX2 (lane 2). Protein extracts immunoprecipitated with anti-HA beads, and revealed with anti-HA and anti-ASF/SF2 antibodies. (b) Same as (a) with pHA-miniCDX2 instead of pHA-CDX2. (E) Interaction between SRp30c and CDX2 or miniCDX2. Same as (D) except that protein detection used anti-SRp30c instead of anti-ASF2/SF2 antibody. (F) Effect of CDX2 and miniCDX2 on the interaction between ASF/SF2 and SRp30c. (a) HCT116 cells were transfected with the control plasmid pCMV2-Flag (lane 1), with pHA-CDX2 (lane 2) or pHA-miniCDX2 (lane 3). Cell extracts were immunoprecipitated with anti-ASF/SF2 antibody, and revealed with anti-ASF/SF2 and anti-SRp30c antibodies. (b) Quantification of immunoprecipitated SRp30c relative to ASF/SF2
Discussion
This work reports a number of novel exon–exon junctions for genes expressed in the gut, which deserves future studies of the relevance of RNA splicing in intestinal development, homeostasis and disease. Focusing on the homeobox gene CDX2, a major regulator of gut ontogenesis and function,4, 8, 28 we identified the miniCDX2 variant, resulting from the combination of alternative pre-mRNA splicing and alternative translation initiation. This variant fine-tunes CDX2 activity through versatile functions regarding the full-length homeoprotein: miniCDX2 antagonizes the transcriptional activity of CDX2 by DNA-binding competition, it is neutral with respect to the inhibitory function exerted by CDX2 on β-catenin/Tcf4 signaling, and it has the same activities as CDX2 on cell proliferation, DNA repair and RNA splicing through P27, KU70/80 and splicing factors, respectively. The production of miniCDX2 is based on a conserved sequence motif among CDX2 paralog in vertebrates, that is absent in other members of the caudal family, with the exception of Cdx1b in teleost fishes. Interestingly, in teleosts, the CDX2-type gene has been lost and replaced by a duplicated version of CDX1 (Cdx1b in D. rerio), which is functionally equivalent to the mammalian CDX2 gene for intestinal development.31 Thus, Cdx1b, unlike Cdx1a, may have acquired a similar motif as CDX2, although being a gene of the CDX1 type. The conserved motif of the CDX2-type genes has implications for translation initiation. Indeed, in the context of the CDX2 mRNA, the AUG2 codon used for CDX2 translation is preceded by the upstream and out-of-frame AUG1 that could potentially initiate the synthesis of a 13-aa peptide. Although the existence of this peptide remains elusive, short upstream open reading frames are relatively frequent and can control the translation of the downstream open reading frame or even be involved in diseases.32
In the gut, both the loss or the excessive expression of CDX2 are lethal,8, 9 highlighting the need for a precise regulation of the activity of the gene. Previous studies have already reported transcriptional33, 34 and post-translational levels of regulation.35, 36 This study adds a novel post-transcriptional level of regulation through the production of the variant miniCDX2 that fine-tunes the activity of CDX2. Another variant of CDX2 has been reported, CDX2-A/S, described as a regulator of splicing.37 The present study further uncovers that the transcription factor CDX2 and its variant miniCDX2 also act as splicing regulators through a non-transcriptional mechanism involving their interaction with splicing factors. This involvement in pre-mRNA splicing might considerably broaden the sphere of activity of the CDX2 homeobox gene. Interestingly, miniCDX2 is expressed in the crypts, like the S60-phosphorylated form of CDX2 characterized by a reduced transcriptional activity.36 This suggests that combined mechanisms act to prevent excessive activity of the CDX2 homeoprotein in stem/progenitor cells and hence the premature cell differentiation known to result from excessive CDX2 in the crypts.9
CDX2 has a role in the patterning of the intestine.38 Here, overexpressing miniCDX2 leads to extend the BG territory, which is accompanied by an increase of PDX1, a homeobox gene of the paraHox cluster, like CDX2, involved in BG morphogenesis.29 This suggests that PDX1, together with the CDX2/miniCDX2 balance, participate in shaping the gastric-intestinal boundary, in that the BG territory would depend on a low activity of CDX2 in the proximal small intestine, whereas miniCDX2 overexpression, through DNA-binding competition with CDX2, would allow extending the territory of these glands. In the long term, miniCDX2 overexpression in the BGs leads to brunneroma in which the production of EGF is increased and activates the EGFR in the overlaying crypts. Interestingly, these polyps exhibit gastric-type differentiation and even squamous stratified differentiation, but do not spontaneously progress in cancer, like most brunneroma in man. In line with this, the foregut-type heteroplasia developing in the cecum of Cdx2+/− mice3 and the cecal gastric-type lesions generated by conditional Cdx2 knockout in the adult gut28 also fail to spontaneously progress to malignancy. Thus, the overexpression of the dominant-negative variant miniCDX2 and the loss of function of CDX2 do not represent oncogenic events per se. Yet, CDX2 has a tumor suppressor activity in the gut, as assessed by model studies in mice12, 13 and observations made in colon cancer patients.10, 39 Here, the data showing a modified CDX2/miniCDX2 ratio in favor of miniCDX2 in intestinal cancer open the possibility that miniCDX2, through its dominant-negative effect exerted on the tumor suppressor CDX2, may contribute to disease progression.
Here, we uncovered and characterized an original variant of the CDX2 homeobox gene, which is a major player of intestinal morphogenesis, homeostasis and also involved in gut cancer. The production of this variant represents not only a novel level of regulation of this gene, but also a new way to fine-tune its biological activity through the versatile functions exerted by the truncated variant compared to the full-length homeoprotein. As in the case of another important transcription factor, SOX9,40 this study highlights the relevance of generating protein diversity through alternative splicing in the gut and its diseases.
Materials and Methods
Mice and generation of transgenic animals
Mice were handled according to the protocol approved by the Committee on the Ethics of Animal Experiments of the University of Strasbourg (CREMEAS, C2EA-35) under the permit number AL/43/50/02/13.
To generate jojo-Flag-miniCDX2 mice, the Flag-miniCDX2 sequence of plasmid pFlag-miniCDX2 (see below) was PCR-amplified using the primers AAAAGTCGACTACCATGGACTACAAAGACGATGA/AAAAGTCGACTCAGCCTGGAATTGCTCTGCCG containing each a SalI restriction site. The resulting SalI fragment was inserted in the XhoI site of the vector jojo41 to get plasmid jojo-Flag-miniCDX2. The eluted 8010 bp SalI fragment containing the transgene was injected in mouse eggs at the Mouse Clinic Institute (Illkirch, France). Mice were PCR-genotyped with primers 39/hC2E3R (AGTCATAGCTGTCCCTCTTC/TCAGCCTGGAATTGCTCTGC).
Cdx2+/−,42 VilCre43 and ApcΔ14/+ mice44 have been described. Sox2Cre mice were obtained from The Jackson Laboratory (Charles River, L'Arbresle, France).
Cell lines and colony formation assays
Human colon cancer cell lines Caco2TC7,45 HCT116,46 SW48047 and T84,48 and human embryonic kidney cells HEK29349 were grown as recommended. Colony formation assays were performed on HCT116 cells transfected with the indicated plasmids, as previously described.35
Plasmids
pFlag-CDX2 and pFlag-miniCDX2
RNA extracted from Caco2TC7 cells was subjected to RT-PCR using Platinum TaqPCRx DNA polymerase (Promega Inc., Madison, WI, USA) with the primers AAAAAAGCTTTACGTGAGCTACCTCCTGGACAAGG and AAAATCTAGATCAGCCTGGAATTGCTCTGC to amplify the human CDX2 coding sequence, and with the primers AAAAAAGCTTGTGAGGTCTGCTCCCGGACCCTCGC and AAAATCTAGATCAGCCTGGAATTGCTCTGC to amplify the miniCDX2 coding sequence. PCR fragments were inserted in pCRII-TOPO (TA cloning kit, Life Technologies Invitrogen, Villebon sur Yvette, France), giving, respectively, pCRII-TOPO-CDX2 and pCRII-TOPO-miniCDX2. These plasmids were cut with HindIII and the fragments inserted in the HindIII site of pFlag-CMV2 (Sigma-Aldrich, St. Louis, MO, USA) to get the plasmids pFlag-CDX2 and pFlag-miniCDX2, respectively.
pATG1-ATG2 and pATG1m-ATG2
To construct pATG1-ATG2, the 5′-untranslated region of the mouse Cdx2 gene up to the codon ATG2 was PCR-amplified from pSL1190-9.4733 with the primers TTTTAAGCTTAAGGCCGCTGGCCTGGGACTCCGCGA/TTTTCCATGGTGGCGAGGGACCCAGAGCAGA containing, respectively, HindIII and NcoI sites. The PCR fragment was cut with HindIII and NcoI, and inserted in the corresponding sites of pGl3-control (Promega Inc.) to give pATG1-ATG2. pATG1m-ATG2 was derived from pATG1-ATG2 by site-directed mutagenesis using the Quick Change IIXL Site Directed Mutagenesis Kit (BD Biosciences, San Rose, CA, USA) with the primer TCAACGTTTGTCCCCAGACACCTAGGTGAGGTCTGCTCTGGGT to change the ATG1 start codon into TAG.
pATG1 and pATG1m
Plasmid pATG1 containing the 5′-untranslated region of the murine Cdx2 gene up to the codon ATG1 was constructed as above by PCR amplification with the primers TTTTAAGCTTAAGGCCGCTGGCCTGGGACTCCGCGA and TTTTCCATGGTGTCTGGGGACAAACGTTGT in which the sequence was changed for one nucleotide to create a NcoI cloning site. After insertion of the PCR fragment in the HindIII and NcoI sites of pGl3-control, the correct sequence was restored by point mutagenesis with the primer TTTGTCCCCAGACAGCATGGAAGACGCCAAAAACAT to give pATG1. pATG1m was derived from pATG1 by mutation with the primer TTTGTCCCCAGACACCTAGGAAGACGCCAAAAACAT to change the ATG1 start codon into TAG.
eC2I1 and eC2I1m
The human CDX2 minigene overlapping the exon-1, the intron-1 and the fused exon-2/-3 and having the Flag coding sequence inserted before the Stop codon in the exon-3 was sequentially constructed using three overlapping fragments generated by PCR using Platinum TaqPCRx DNA Polymerase (Life Technologies Invitrogen): the fragment A and B were, respectively, amplified by PCR from human genomic DNA with the primers Af (GTTTAAACTTAAGCTTGCTCCGCACGCCAGCCTGT)/Ar (TCACAGGTCAGAGGTTCAGAG), and with the primers Bf (CTCTGAACCTCTGACCTGTGA)/Br (TCCGTGTACACCACTCGATATTT); the fragment C was amplified from pFlag-CDX2 DNA using the primers Cf (AGTGGTGTACACGGACCACC) and Cr (GCCCTCTAGACTCGAGTCACTTGTCGTCATCGTCTTTGTAGTCCTGGGTGACGGTGGGGTTTA). Af contains 15 nucleotides complementary to the cloning site of the vector pcDNA4/TO, followed by 20 nucleotides complementary to the 5′ extremity of the CDX2 gene; Cr contains 15 nucleotides complementary to the cloning site of pcDNA4/TO, followed by a translation Stop codon, 24 nucleotides complementary to the Flag sequence and 20 nucleotides complementary to the CDX2 gene; the primers Ar/Bf are partially complementary over 15 nucleotides, as the primers Br/Cf. The three PCR fragments A, B and C and the vector pcDNA4/TO cut with HindIII and XhoI were purified by agarose gel electrophoresis, mixed and incubated with the In-Fusion HD Cloning kit (Clontech Laboratories, Inc., Mountain View, CA, USA). The resulting plasmid eC2I1 was verified by sequencing. eC2I1m was derived from eC2I1 using the primer ACCCTCGCCACCATGAACGTGAGCTACCTCC to change the GT splicing site into GA.
Other plasmids were: pS33A-β-catenin encoding activated β-catenin,50 pcDNA3-TCF4E-myc encoding Myc-tagged Tcf4,51 the reporter plasmids TOPFlash,52 pSI-Luc,53 pHis-SRp30c and pHis-ASF/SF2,54 and commercially-available pEGFP (Clontech Laboratories Inc.), pGl3-basic, pGl3-control, pRL-Null (Promega Inc.) and pCMV-Flag2 (Sigma-Aldrich).
Antibodies
The antibody against the miniCDX2 protein was obtained by immunizing rabbits with the synthetic peptide MVRSAPGPSPP-cystein coupled to KLH (Eurogentech SA, Seraing, Belgium). C2T anti-peptide antibody was purified by affinity chromatography. The peptide was reduced by TCEP (Tris-(2-carboxyethyl) phosphine) using Immobilized TCEP-Disulfide Reducing gel (Pierce Biotechnology, Rockford, lL, USA). Reduced peptide of 2.5 mg were incubated with 2 ml sulfonyl-agarose (SulfoLink Trial kit, Pierce Biotechnology) during 1 h and the affinity gel was equilibrated in Tris 50 mM/NaCl 0.15 M pH 7.3 buffer. Rabbit immune serum of 2 ml were passed through the affinity column, washed with Tris 50 mM/NaCl 0.15–0.5 M pH 7.3. Specific immunoglobulins were eluted with glycine 0.2 M pH 2.7 and neutralized with 50 μl Tris buffer 1 M pH 8. The C2T antibody raised against the Nter part of miniCDX2 was used at 15 μg/ml.
Commercial antibodies were against: actin (clone C4, Millipore, St. Quentin-en-Yvelines, France), ASF/SF2 (sc-33652, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), β-catenin (mouse clone 14, BD Transduction Lab, San Rose, CA, USA), CDX2 (mouse clone 392M, BioGenex, San Ramon, CA, USA), Claudin-18 (Life Technologies Invitrogen), Flag (mouse clone M2, Sigma-Aldrich), GFP (BD Biosciences, Franklin Lakes, NJ, USA), HA (clone 3F10, Roche Applied Science, Meylan, France), KU70 (rabbit clone EPR4027, Abcam, Cambridge, MA, USA), KU80 (rabbit clone EPR3468, Abcam), Myc (mouse clone 9E10, Santa Cruz Biotechnology Inc.), p27KIP1 (mouse clone 57, BD Transduction Lab), PDX1 (rabbit, Abcam ab47267), phospho-EGFR (Cell Signaling Technology, Ozyme, Montigny-le-Bretonneux, France), phospho-histone-3 (Ser10 mitosis marker, Millipore Upstate, Saint-Quentin Yvelines, France) and SRp30c (sc-134046, Santa Cruz Biotechnology Inc.).
Cellular RNA preparation and in vitro RNA transcription
RNA was prepared using TRI Reagent (Life Technologies Ambio, Villebon sur Yvette, France) and treated with DNAse (RQ1 RNAse-free DNAse, Promega Inc.).
For in vitro transcription of capped and uncapped RNA, DNA templates were amplified using Platinum TaqPCRx DNA polymerase from the plasmids pATG1, pATG1-ATG2, pATG1m-ATG2 and pGL3-control with Tption-f/Tption-r (TTTTTTTTTTTAATACGACTCACTATAGGGAGAAGGCCTAGGCTTTTGCAAAAAGCTT/(T)42CACTGCATTCTAGTTGTG) containing, respectively, the T7 promoter and polydT sequences. After purification (QIAquick PCR Purification kit, Qiagen, Venio, The Netherlands), 1 μg of each PCR fragment was in vitro transcribed with or without m7G(5′)ppp(5′)G RNA Cap (S1404S, BioLabs, Ipswich, MA, USA) in the ratio of 1 GTP/4 GTPcap, in 25 μl using HiScribe T7 in vitro Transcription kit (BioLabs). After treatment with DNAse (RQ1 RNAse-free DNAse, Promega Inc.), RNAs were precipitated in NH4-acetate 2.5 M on ice, washed in 70% ethanol and solubilized in 5 μl RNAse-free water.
RNAseq analysis
RNA integrity was checked on nano RNA chips with a Bioanalyser 2100 (Agilent Technologies, Santa Clara, CA, USA) and no sign of RNA degradation was detected. Total RNA of 1 μg was used to construct the mRNAseq libraries with Illumina’s TruSeq RNA sample kit following manufacturer’s instructions (Illumina, San Diego, CA, USA). Libraries were validated and quantified on DNA1000 chips and a Bioanalyser 2100 (Agilent Technologies, Les Ulis, France). Paired-end reads of 50 bp were obtained with a HiSeq1000 by multiplexing three libraries on one lane. Demultiplexing and generation of raw fastq files were performed with CASAVA v1.7. Mapping against the reference mouse genome GRCm38 was performed with tophat 2.0.11 and bowtie2 using the options –b2-sensitive -a 5 -p 5 –library-type fr-unstranded -r 180 –mate-std-dev 80. Quantification of the reads was performed with HTSeq and DESeq2 using the reference gene annotation from ensembl v75. Genes with >10 normalized reads in the three replicates as given in DESeq2 were considered as significantly detected. De novo transcripts assembly was performed with cufflinks v2.2.1 with the options -u –library-type fr-firststrand, and providing the reference genome fasta file and gene annotation from ensembl v75. Search of novel exon–exon jonctions was made with cuffcompare and consensus novel junctions found in all biological triplicates obtained with bedtools. The resulting bed file was then imported in R as a Grange object for gene annotation.
Northern blots, RT-PCR and RT-qPCR
PolyA+ RNA (PolyATract System, Promega Inc.) was separated by electrophoresis on 2% agarose/2 M formaldehyde gels (10 μg polyadenylated RNA/lane) and transferred to nylon membranes. Hybridization and revelation was performed as described55 with 100 ng/ml of DIG-labeled antisense cRNA probe. This probe overlapped the 3′-untranslated region of the exon-3 of the human CDX2 gene, and was in vitro transcribed by T7 RNA polymerase from plasmid pCRII-TOPO-miniCDX2 linearized with BsrGI. A DIG-labeled human GAPDH probe was used for normalization.
Reverse transcription was performed with 2 μg RNA using SuperScript II Reverse Transcriptase (Life Technologies, Invitrogen) and Oligo(dT)12-18 primer (Invitrogen).
RT-PCR used 1/10th of the RT solution. Concomitant amplification of the two splicing forms of the human CDX2 gene was performed with primers CDX21F/CCR (GCAGCCTCCAGCGTCGGTC/TCAGCCTGGAATTGCTCTGC) for 35 cycles (30 s at 95 °C, 30 s at 55 °C and 1.5 min at 68 °C). Specific amplification of the mouse CDX2 mRNA and miniCDX2 mRNA, respectively, used the primer pairs mhC2Flf/mhC2E3r (CCCTCGCCACCATGTACG/CTCTGCGGTTCTGAAACCA) and mhC2Tqf (CCCTCGCCACCATTGAAAA)/mhC2E3r.
For RT-qPCR, 2 μg of mouse intestinal RNA, of RNA from human colon biopsies or from human colon cancer Caco2TC7 cells were reverse transcribed in 20 μl using the AMV Reverse Transcriptase kit (Life Technologies, Invitrogen) and oligo-dT. RT-qPCR was performed on 1 μl of RT reaction in 20 μl using Syber Green PCR Master Mix (Life Technologies Applied Biosystems, Villebon sur Yvette, France) and 7500 Real-time PCR System (Life Technologies Applied Biosystems). For mouse RNA, the cycles were 95 °C 15 s, 57 °C 1 min, with the primers: CCCTCGCCACCATTGAAAA/GTGATGTATCGACTAAAGTG for miniCDX2, CTAGGAAGCCAAGTGAAAAC/GTGATGTATCGACTAAAGTG for CDX2 and CCCCACAACTCTTCCATTCT/GCAGGAGTGATAGGGGTCAT for TBP. For Caco2TC7 cell RNA, the cycles were 95 °C 15 s, 68 °C 1 min with the primers: CCCTCGCCACCATTGAAAA/TGCCTCTCAGAGAGCCCCAGCGTGG for miniCDX2, CTCGGCAGCCAAGTGAAAAC/GTGATGTAGCGACTGTAGTG for CDX2 and TGCACAGGAGCCAAGAGTGAA/CACATCACAGCTCCCCACCA for TBP.
Quantification of the SI, MMP7, P27KIP1, EGF and TBP transcripts was performed by RT-qPCR using diluted RT products (5–10 ×) mixed with TaqMan Master Mix, gene-specific TaqMan probe and the primers sets (TaqMan Gene Expression assays, Life Technologies Applied Biosystems): SI, Hs00356112_m1; MMP7, Hs01042795_m1; P27KIP1, Hs01597588_m1; EGF, Hs01099999_m1; TBP, Hs99999910_m1.
Analysis of the results (triplicates) was performed with the 7500 software v2.0.1 (Life Technologies Applied Biosystems) using the relative ΔΔCt quantification method.
DNA and RNA transfections and luciferase assays
Plasmid DNA transfection was performed during 48 h with JetPEI (Polyplus Transfection, Illkirch, France). RNA transfection was performed for 3 h with 100–500 ng of in vitro-transcribed RNA (see below) using 0.25–1.25 μl TransIT mRNA and 0.375–1.875 μl of boost (Mirus Bio, Madison, WI, USA) on cells at 80% confluence. Firefly and Renilla luciferase activities were measured at least three times in triplicate using a dual reporter luciferase assay (Promega Inc.) in cell extracts prepared 48 h after DNA transfection or 3 h after RNA transfection. Means are given ±S.D.
Immunostaining
Immunochemical staining and/or immunofluorescence detection were performed on paraffin-embedded tissue sections.33 Histochemical staining used the Vectastain ABC kit (Vector Labs, Peterborough, UK) and DAB 0.3 mg/ml/H2O2 0.03%. Double-immunofluorescence staining used secondary Alexa488-labeled anti-mouse antibody and Alexa688-labeled anti-rabbit antibody (Life Technologies Invitrogen) coupled to fluorescence amplification with the TSA Fluorescent Plus System (Perkin Elmer, Waltham, MA, USA).
Co-immunoprecipitations and western blots
Transfected cells were lysed in buffer containing 50 mM Tris buffer pH 7.4, 150 mM NaCl, 1 mM EDTA and protease inhibitor cocktail (Roche Diagnostic, Mannheim, Germany). The buffer additionally contained 1% Triton X-100 for β-catenin and Flag immunoprecipitation, or 1% Triton X-100 and 1% NP-40 for ASF/SF2 immunoprecipitation, or 1% NP-40 for HA immunoprecipitation. Lysates were cleared by centrifugation at 12 000 × g for 20 min at 4 °C, and the protein extracts were then treated with 1 μl of 0.1 mg/ml RNaseA for 30 min on ice when indicated. Immunoprecipitation was performed using 1 mg of lysate supernatant incubated overnight at 4 °C with gentle rocking in 1 ml of lysis buffer with the appropriate antibody: anti-Flag antibody (5 μg of clone M2, Sigma-Aldrich), anti-β-catenin antibody (1 μg of clone 14, BD Biosciences), anti-HA antibody (20 μl of agarose beads coupled to clone 3F10, Roche Applied Science) and anti-ASF/SF2 antibody (3 μg of sc-33652, Santa Cruz Biotechnology Inc.). After adding 25 μl of Protein-G-agarose beads (Roche Applied Science) for 2 h at 4 °C, the beads were washed in lysis buffer. The immunoprecipitated material was eluted using SDS-PAGE sample loading buffer and analyzed by western blots performed as described.35
Chromatin immunoprecipitations
Transfected HCT116 cells were fixed with 1% (v/v) formaldehyde for 10 min at RT and quenched with 0.125 M glycine for 5 min. ChIPs were carried out using the EZ-Magna ChIP G Chromatin Immunoprecipitation kit (Upstate Millipore, Billerica, MA, USA). Sheared cross-linked chromatin from ~106 cells was incubated overnight at 4 °C with 1 μg of normal mouse IgG (Upstate Millipore) or the appropriate antibody. Input corresponds to non-immunoprecipitated sheared cross-linked chromatin from 105 cells. PCR analysis was performed with 1/25th of immunoprecipitated DNA as template. The primers used for PCR amplification of three human targets of the CDX2 protein were: SI promoter GGCTGGTAAGGGTGCAATAA/GCCTGTTCTCTTTGCTATGTTG; Muc2 promoter: GTGTGTTGGCATTCAGGCTA/AGGGTAGGAGGGCAGGAAG; Li-cad promoter: TTTCAAAAGCAGGCACAGCC/AGTGGTCGAGACTCTTGCTAC.
In vitro DNA repair assays
Cell-free extracts were prepared as follows. A total of 3 × 107 transfected cells were washed in cold PBS, suspended in four packed cell volumes of cold hypotonic lysis buffer (10 mM Tris-HCl pH 7.6, 1 mM DTT, 5 mM MgCl2, 1 mM EDTA and protease inhibitors), incubated for 40 min on ice and disrupted using a Dounce homogenizer (40 strokes). Sucrose was added to reach 250 mM. Extracts were centrifuged 10 min at 1000 × g to discard cell debris and washed again in hypotonic lysis buffer containing 250 mM sucrose. Nuclear pellet was then suspended in two volumes of nuclear extraction buffer (20 mM Tris-HCl pH 7.6, 1 mM DTT, 2 mM EDTA, 20% glycerol, 500 mM NaCl and protease inhibitors) and incubated for 30 min on ice. Nuclear extracts were clarified by 30 min centrifugation at 21 000 × g and dialyzed overnight against 20 mM Tris-HCl pH 7.6, 1 mM EDTA, 1 mM DTT, 20% glycerol, 25 mM NaCl and 0.2 mM PMSF.
End-joining reaction was performed in 30 μl by incubating 50 ng of BamHI-linearized pcDNA3 plasmid with 5 mg of nuclear extract for 1 h at 25 °C in 50 mM Tris-HCl pH 7.6, 5 mM MgCl2, 1 mM ATP, 1 mM DTT, 50 mM dNTP, 80 mM NaCl and protease inhibitors. Reaction was stopped by RNAse treatment (0.25 mg/ml of RNAse for 10 min at 37 °C), followed by proteinase K (0.5% SDS, 50 mM EDTA and 1 mg/ml of proteinase K at 37 °C for 1 h). DNA was purified by phenol and chloroform extraction, recovered by ethanol precipitation and analyzed by electrophoresis on a 0.85% agarose gel.
Statistical analyses
Experiments were performed in at least three independent biological replicates in triplicates. P-value calculation indicated significant differences tested by the Wilcoxon–Mann–Whitney test.
References
Clevers H . The intestinal crypt, a prototype stem cell compartment. Cell 2013; 154: 274–284.
Li H, Jasper H . Gastrointestinal stem cells in health and disease: from flies to humans. Dis Model Mech 2016; 9: 487–499.
Beck F, Chawengsaksophak K, Waring P, Playford RJ, Furness JB . Reprogramming of intestinal differentiation and intercalary regeneration in cdx2 mutant mice. Proc Natl Acad Sci USA 1999; 96: 7318–7323.
Gao N, White P, Kaestner KH . Establishment of intestinal identity and epithelial-mesenchymal signaling by Cdx2. Dev Cell 2009; 16: 588–599.
San Roman AK, Tovaglieri A, Breault DT, Shivdasani RA . Distinct processes and transcriptional targets underlie CDX2 requirements in intestinal stem cells and differentiated villus cells. Stem Cell Rep 2015; 5: 673–681.
Simmini S, Bialecka M, Huch M, Kester L, van de Wetering M, Sato T et al. Transformation of intestinal stem cells into gastric stem cells on loss of transcription factor Cdx2. Nat Commun 2014; 5: 5728.
Stringer EJ, Duluc I, Saandi T, Davidson I, Bialecka M, Sato T et al. Cdx2 determines the fate of postnatal intestinal endoderm. Dev Camb Engl 2012; 139: 465–474.
Verzi MP, Shin H, Ho L-L, Liu XS, Shivdasani RA . Essential and redundant functions of caudal family proteins in activating adult intestinal genes. Mol Cell Biol 2011; 31: 2026–2039.
Crissey MAS, Guo R-J, Funakoshi S, Kong J, Liu J, Lynch JP . Cdx2 levels modulate intestinal epithelium maturity and Paneth cell development. Gastroenterology 2011; 140: 517–528.e8.
Bae JM, Lee TH, Cho N-Y, Kim T-Y, Kang GH . Loss of CDX2 expression is associated with poor prognosis in colorectal cancer patients. World J Gastroenterol 2015; 21: 1457–1467.
De Sousa E, Melo F, Wang X, Jansen M, Fessler E, Trinh A et al. Poor-prognosis colon cancer is defined by a molecularly distinct subtype and develops from serrated precursor lesions. Nat Med 2013; 19: 614–618.
Aoki K, Tamai Y, Horiike S, Oshima M, Taketo MM . Colonic polyposis caused by mTOR-mediated chromosomal instability in Apc+/Delta716 Cdx2+/- compound mutant mice. Nat Genet 2003; 35: 323–330.
Bonhomme C, Duluc I, Martin E, Chawengsaksophak K, Chenard M-P, Kedinger M et al. The Cdx2 homeobox gene has a tumour suppressor function in the distal colon in addition to a homeotic role during gut development. Gut 2003; 52: 1465–1471.
Gross I, Duluc I, Benameur T, Calon A, Martin E, Brabletz T et al. The intestine-specific homeobox gene Cdx2 decreases mobility and antagonizes dissemination of colon cancer cells. Oncogene 2008; 27: 107–115.
Hryniuk A, Grainger S, Savory JGA, Lohnes D . Cdx1 and Cdx2 function as tumor suppressors. J Biol Chem 2014; 289: 33343–33354.
Kelemen O, Convertini P, Zhang Z, Wen Y, Shen M, Falaleeva M et al. Function of alternative splicing. Gene 2013; 514: 1–30.
Nilsen TW, Graveley BR . Expansion of the eukaryotic proteome by alternative splicing. Nature 2010; 463: 457–463.
Chepelev I, Chen X . Alternative splicing switching in stem cell lineages. Front Biol 2013; 8: 50–59.
Cieply B, Carstens RP . Functional roles of alternative splicing factors in human disease. Wiley Interdiscip Rev RNA 2015; 6: 311–326.
Muñoz J, Stange DE, Schepers AG, van de Wetering M, Koo B-K, Itzkovitz S et al. The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent ‘+4’ cell markers. EMBO J 2012; 31: 3079–3091.
Kozak M . An analysis of 5’-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res 1987; 15: 8125–8148.
Kozak M . Pushing the limits of the scanning mechanism for initiation of translation. Gene 2002; 299: 1–34.
Stoneley M, Spencer JP, Wright SC . An internal ribosome entry segment in the 5′ untranslated region of the mnt gene. Oncogene 2001; 20: 893–897.
Freund JN, Duluc I, Reimund JM, Gross I, Domon-Dell C . Extending the functions of the homeotic transcription factor Cdx2 in the digestive system through nontranscriptional activities. World J Gastroenterol 2015; 21: 1436–1443.
Guo R-J, Funakoshi S, Lee HH, Kong J, Lynch JP . The intestine-specific transcription factor Cdx2 inhibits beta-catenin/TCF transcriptional activity by disrupting the beta-catenin-TCF protein complex. Carcinogenesis 2010; 31: 159–166.
Aoki K, Kakizaki F, Sakashita H, Manabe T, Aoki M, Taketo MM . Suppression of colonic polyposis by homeoprotein CDX2 through its nontranscriptional function that stabilizes p27Kip1. Cancer Res 2011; 71: 593–602.
Renouf B, Soret C, Saandi T, Delalande F, Martin E, Vanier M et al. Cdx2 homeoprotein inhibits non-homologous end joining in colon cancer but not in leukemia cells. Nucleic Acids Res 2012; 40: 3456–3469.
Stringer EJ, Duluc I, Saandi T, Davidson I, Bialecka M, Sato T et al. Cdx2 determines the fate of postnatal intestinal endoderm. Development 2012; 139: 465–474.
Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA et al. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development 1996; 122: 983–995.
Powell AE, Wang Y, Li Y, Poulin EJ, Means AL, Washington MK et al. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell 2012; 149: 146–158.
Flores MV, Hall CJ, Davidson AJ, Singh PP, Mahagaonkar AA, Zon LI et al. Intestinal differentiation in zebrafish requires Cdx1b, a functional equivalent of mammalian Cdx2. Gastroenterology 2008; 135: 1665–1675.
Wethmar K, Smink JJ, Leutz A . Upstream open reading frames: molecular switches in (patho)physiology. BioEssays News Rev Mol Cell Dev Biol 2010; 32: 885–893.
Benahmed F, Gross I, Gaunt SJ, Beck F, Jehan F, Domon-Dell C et al. Multiple regulatory regions control the complex expression pattern of the mouse Cdx2 homeobox gene. Gastroenterology 2008; 135: 1238–1247.
Saandi T, Baraille F, Derbal-Wolfrom L, Cattin AL, Benahmed F, Martin E et al. Regulation of the tumor suppressor homeogene Cdx2 by HNF4alpha in intestinal cancer. Oncogene 2013; 32: 3782–3788.
Gross I, Lhermitte B, Domon-Dell C, Duluc I, Martin E, Gaiddon C et al. Phosphorylation of the homeotic tumor suppressor Cdx2 mediates its ubiquitin-dependent proteasome degradation. Oncogene 2005; 24: 7955–7963.
Rings EH, Boudreau F, Taylor JK, Moffett J, Suh ER, Traber PG . Phosphorylation of the serine 60 residue within the cdx2 activation domain mediates its transactivation capacity. Gastroenterology 2001; 121: 1437–1450.
Witek ME, Snook AE, Lin JE, Blomain ES, Xiang B, Magee MS et al. A novel CDX2 isoform regulates alternative splicing. PLoS ONE 2014; 9: e104293.
Grainger S, Savory JGA, Lohnes D . Cdx2 regulates patterning of the intestinal epithelium. Dev Biol 2010; 339: 155–165.
Dalerba P, Sahoo D, Paik S, Guo X, Yothers G, Song N et al. CDX2 as a prognostic biomarker in stage II and stage III colon cancer. N Engl J Med 2016; 374: 211–222.
Abdel-Samad R, Zalzali H, Rammah C, Giraud J, Naudin C, Dupasquier S et al. MiniSOX9, a dominant-negative variant in colon cancer cells. Oncogene 2011; 30: 2493–2503.
Collombat P, Hecksher-Sorensen J, Krull J, Berger J, Riedel D, Herrera PL et al. Embryonic endocrine pancreas and mature beta cells acquire alpha and PP cell phenotypes upon Arx misexpression. J Clin Invest 2007; 117: 961–970.
Chawengsaksophak K, James R, Hammond VE, Kontgen F, Beck F . Homeosis and intestinal tumours in Cdx2 mutant mice. Nature 1997; 385: 84–87.
El Marjou F, Janssen KP, Chang BH, Li M, Hindie V, Chan L et al. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 2004; 39: 186–193.
Colnot S, Niwa-Kawakita M, Hamard G, Godard C, Le Plenier S, Houbron C et al. Colorectal cancers in a new mouse model of familial adenomatous polyposis: influence of genetic and environmental modifiers. Lab Invest 2004; 84: 1619–1630.
Chantret I, Rodolosse A, Barbat A, Dussaulx E, Brot-Laroche E, Zweibaum A et al. Differential expression of sucrase-isomaltase in clones isolated from early and late passages of the cell line Caco-2: evidence for glucose-dependent negative regulation. J Cell Sci 1994; 107: 213–225.
Brattain MG, Fine WD, Khaled FM, Thompson J, Brattain DE . Heterogeneity of malignant cells from a human colonic carcinoma. Cancer Res 1981; 41: 1751–1756.
Leibovitz A, Stinson JC, McCombs WB III, McCoy CE, Mazur KC, Mabry ND . Classification of human colorectal adenocarcinoma cell lines. Cancer Res 1976; 36: 4562–4569.
Murakami H, Masui H . Hormonal control of human colon carcinoma cell growth in serum-free medium. Proc Natl Acad Sci USA 1980; 77: 3464–3468.
Graham FL, Smiley J, Russell WC, Nairn R . Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol 1977; 36: 59–74.
Aberle H, Bauer A, Stappert J, Kispert A, Kemler R . Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J 1997; 16: 3797–3804.
Tetsu O, McCormick F . Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 1999; 398: 422–426.
van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J et al. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 1997; 88: 789–799.
Rodolosse A, Chantret I, Lacasa M, Chevalier G, Zweibaum A, Swallow D et al. A limited upstream region of the human sucrase-isomaltase gene confers glucose-regulated expression on a heterologous gene. Biochem J 1996; 315: 301–306.
Paradis C, Cloutier P, Shkreta L, Toutant J, Klarskov K, Chabot B . hnRNP I/PTB can antagonize the splicing repressor activity of SRp30c. RNA 2007; 13: 1287–1300.
Lefebvre O, Sorokin L, Kedinger M, Simon-Assmann P . Developmental expression and cellular origin of the laminin alpha2, alpha4, and alpha5 chains in the intestine. Dev Biol 1999; 210: 135–150.
Acknowledgements
This work was supported by the INSERM (France) and the Fondation ARC (grants 3759 and 4872). CB, AN and CS were funded by the MRES (France). CB and CS were further supported by the Ligue Contre le Cancer, and TS by the Worldwide Cancer Research, UK (08-0199). We thank Dr. MA Birling and the Mouse Clinic Institute for generating the jojo-Flag-miniCdx2 mice, Professor MP Chenard (CHU de Strasbourg, France) for pathological evaluation of the mice, Dr. S Robine (CNRS, UMR 144, Institut Curie, Paris) for the VilCre mice, Professor B Chabot (Université de Sherbrooke) for the plasmids pHis-SRp30c and pHis-ASF/SF2, and Dr. JF Launay (Inserm U682, Strasbourg) for help in C2T antibody purification. The data described here have been deposited in the GEO data base under the access code GSE89992. The Genbank accession number of human miniCDX2 is KJ531444.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by D Aberdam
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Rights and permissions
About this article
Cite this article
Balbinot, C., Vanier, M., Armant, O. et al. Fine-tuning and autoregulation of the intestinal determinant and tumor suppressor homeobox gene CDX2 by alternative splicing. Cell Death Differ 24, 2173–2186 (2017). https://doi.org/10.1038/cdd.2017.140
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cdd.2017.140
This article is cited by
-
Microarray analysis reveals ONC201 mediated differential mechanisms of CHOP gene regulation in metastatic and nonmetastatic colorectal cancer cells
Scientific Reports (2021)
-
Determining the prognostic significance of alternative splicing events in soft tissue sarcoma using data from The Cancer Genome Atlas
Journal of Translational Medicine (2019)
-
Severe head dysgenesis resulting from imbalance between anterior and posterior ontogenetic programs
Cell Death & Disease (2019)