Abstract
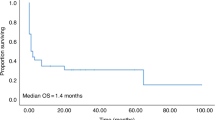
Diabetes mellitus (DM) is a factor in the hematopoietic cell transplantation-comorbidity index. However, the impact of pre-transplant DM on morbidity and cause-specific non-relapse mortality (NRM) remains unclear. We performed a retrospective study with registry data that included a total of 7626 patients who underwent their first allogeneic hematopoietic SCT (HSCT) between 2007 and 2010. The median age was 44 years (range 0–88). Compared with patients without pre-transplant DM (non-DM group, n=7248), patients with pre-transplant DM (DM group, n=378) were older and were more likely to have high-risk disease, a reduced-intensity conditioning regimen and GVHD prophylaxis using tacrolimus. Multivariate analyses showed that pre-transplant DM was associated with increased risks of NRM (hazard ratio (HR)1.46, 95% confidence interval (CI) 1.21–1.76, P<0.01) and infection-related NRM (HR 2.08, 95% CI 1.58–2.73, P<0.01). The presence of pre-transplant DM was associated with an increased risk of overall mortality in a multivariate analysis (HR 1.55, 95% CI 1.35–1.78, P<0.01). In conclusion, pre-transplant DM was a risk factor for NRM, particularly infection-related mortality, after allogeneic HSCT. To improve the clinical outcome in patients with DM, the benefits of strict infection control and appropriate glycemic control should be explored in future trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kurosawa S, Yakushijin K, Yamaguchi T, Atsuta Y, Nagamura-Inoue T, Akiyama H et al. Recent decrease in non-relapse mortality due to GVHD and infection after allogeneic hematopoietic cell transplantation in non-remission acute leukemia. Bone Marrow Transplant 2013; 48: 1198–1204.
Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med 2010; 363: 2091–2101.
Horan JT, Logan BR, Agovi-Johnson MA, Lazarus HM, Bacigalupo AA, Ballen KK et al. Reducing the risk for transplantation-related mortality after allogeneic hematopoietic cell transplantation: how much progress has been made? J Clin Oncol 2011; 29: 805–813.
Kurosawa S, Yakushijin K, Yamaguchi T, Atsuta Y, Nagamura-Inoue T, Akiyama H et al. Changes in incidence and causes of non-relapse mortality after allogeneic hematopoietic cell transplantation in patients with acute leukemia/myelodysplastic syndrome: an analysis of the Japan transplant outcome registry. Bone Marrow Transplant 2013; 48: 529–536.
Sorror ML, Giralt S, Sandmaier BM, De Lima M, Shahjahan M, Maloney DG et al. Hematopoietic cell transplantation specific comorbidity index as an outcome predictor for patients with acute myeloid leukemia in first remission: combined FHCRC and MDACC experiences. Blood 2007; 110: 4606–4613.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005; 106: 2912–2919.
Derr RL, Hsiao VC, Saudek CD . Antecedent hyperglycemia is associated with an increased risk of neutropenic infections during bone marrow transplantation. Diabetes Care 2008; 31: 1972–1977.
Hammer MJ, Casper C, Gooley TA, O'Donnell PV, Boeckh M, Hirsch IB . The contribution of malglycemia to mortality among allogeneic hematopoietic cell transplant recipients. Biol Blood Marrow Transplant 2009; 15: 344–351.
Fuji S, Kim SW, Mori S, Fukuda T, Kamiya S, Yamasaki S et al. Hyperglycemia during the neutropenic period is associated with a poor outcome in patients undergoing myeloablative allogeneic hematopoietic stem cell transplantation. Transplantation 2007; 84: 814–820.
Gebremedhin E, Behrendt CE, Nakamura R, Parker P, Salehian B . Severe hyperglycemia immediately after allogeneic hematopoietic stem-cell transplantation is predictive of acute graft-versus-host disease. Inflammation 2013; 36: 177–185.
Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation 2002; 106: 2067–2072.
McCowen KC, Malhotra A, Bistrian BR . Stress-induced hyperglycemia. Crit Care Clin 2001; 17: 107–124.
Ginter E, Simko V . Type 2 diabetes mellitus, pandemic in 21st century. Adv Exp Med Biol 2012; 771: 42–50.
Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2095–2128.
Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 2011; 378: 31–40.
Fuji S, Kim SW, Mori S, Kamiya S, Yoshimura K, Yokoyama H et al. Intensive glucose control after allogeneic hematopoietic stem cell transplantation: a retrospective matched-cohort study. Bone Marrow Transplant 2009; 44: 105–111.
Atsuta Y, Suzuki R, Yoshimi A, Gondo H, Tanaka J, Hiraoka A et al. Unification of hematopoietic stem cell transplantation registries in Japan and establishment of the TRUMP System. Int J Hematol 2007; 86: 269–274.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995; 15: 825–828.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974; 18: 295–304.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant 2009; 15: 1628–1633.
Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant 2009; 15: 367–369.
Kanda Y . Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 2013; 48: 452–458.
Rammaert B, Lanternier F, Poirée S, Kania R, Lortholary O . Diabetes and mucormycosis: a complex interplay. Diabetes Metab 2012; 38: 193–204.
Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 2005; 41: 634–653.
Sluik D, Boeing H, Montonen J, Pischon T, Kaaks R, Teucher B et al. Associations between general and abdominal adiposity and mortality in individuals with diabetes mellitus. Am J Epidemiol 2011; 174: 22–34.
Unick JL, Beavers D, Jakicic JM, Kitabchi AE, Knowler WC, Wadden TA et al. Effectiveness of lifestyle interventions for individuals with severe obesity and type 2 diabetes: results from the Look AHEAD trial. Diabetes Care 2011; 34: 2152–2157.
Mercer BN, Morais S, Cubbon RM, Kearney MT . Diabetes mellitus and the heart. Int J Clin Pract 2012; 66: 640–647.
Shikata K, Ninomiya T, Kiyohara Y . Diabetes mellitus and cancer risk: review of the epidemiological evidence. Cancer Sci 2013; 104: 9–14.
Eijgenraam P, Heinen MM, Verhage BA, Keulemans YC, Schouten LJ, van den Brandt PA . Diabetes type II, other medical conditions and pancreatic cancer risk: a prospective study in The Netherlands. Br J Cancer 2013; 109: 2924–2932.
Ong JP, Younossi ZM . Epidemiology and natural history of NAFLD and NASH. Clin Liver Dis 2007; 11: 1–16.
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35: 1364–1379.
Marik PE, Preiser JC . Toward understanding tight glycemic control in the ICU: a systematic review and metaanalysis. Chest 2010; 137: 544–551.
Acknowledgements
This work was supported by grants from the Japanese Ministry of Health, Labor and Welfare and the National Cancer Research and Development Fund. Some of the results were presented at the 32nd Annual Meeting of the Japanese Society of Hematology in Kanazawa, 9 March 2013. We thank the medical, nursing, data-processing, laboratory and clinical staff at the participating centers for their important contributions to this study and their dedicated care of the patients.
Author Contributions
KT participated in research design, data analysis and writing of the paper; SF participated in research design, data analysis and writing of the paper; NU, HO, KO, TE, HS, YM, KK and RS gathered the data; TF participated in research design and writing of the paper. All of the authors approved the submission of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Bone Marrow Transplantation website
Supplementary information
Rights and permissions
About this article
Cite this article
Takano, K., Fuji, S., Uchida, N. et al. Pre-transplant diabetes mellitus is a risk factor for non-relapse mortality, especially infection-related mortality, after allogeneic hematopoietic SCT. Bone Marrow Transplant 50, 553–558 (2015). https://doi.org/10.1038/bmt.2014.315
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2014.315
This article is cited by
-
The impact of pre-transplantation diabetes and obesity on acute graft-versus-host disease, relapse and death after allogeneic hematopoietic cell transplantation: a study from the EBMT Transplant Complications Working Party
Bone Marrow Transplantation (2024)
-
Impact of pre-transplant individual comorbidities on risk of ICU admission and survival outcomes following allogeneic hematopoietic stem cell transplantation
Bone Marrow Transplantation (2023)
-
Clinical impact of hyperglycemia on days 0–7 after allogeneic stem cell transplantation
Bone Marrow Transplantation (2017)
-
Hyperglycemia as a possible risk factor for mold infections—the potential preventative role of intensified glucose control in allogeneic hematopoietic stem cell transplantation
Bone Marrow Transplantation (2017)
-
How do I manage hyperglycemia/post-transplant diabetes mellitus after allogeneic HSCT
Bone Marrow Transplantation (2016)