Abstract
Background:
In the United Kingdom, totally implantable venous access systems (TIVAS) are not routinely used. Compared with Hickman catheters, these devices are more expensive and complex to insert. However, it is unclear whether the higher costs may be offset by perceived greater health benefits. This pilot trial aimed to generate relevant data to inform the design of a larger definitive randomised controlled trial.
Methods:
This was a phase II prospective, randomised, open trial from two UK oncology centres. The primary end point was overall complication rate. Secondary end points included individual complication rates, time to first complication and quality of life. Analysis was by intention to treat. An economic evaluation was also carried out.
Results:
A total of 100 patients were randomised in a 3 : 1 ratio to receive a Hickman or a TIVAS. Overall, 54% of patients in the Hickman arm suffered one or more complications compared with 38% in the TIVAS arm (one-sided P=0.068). In the Hickman arm, 28% of the devices were removed prematurely due to a complication compared with 4% in the TIVAS arm. Quality of life based on the device-specific questionnaire was greater in the TIVAS arm for 13 of the 16 questions. The economic evaluation showed that Hickman arm was associated with greater mean cost per patient £1803 (95% CI 462, 3215), but similar quality-adjusted life years −0.01 (95% CI −0.15, 0.15) than the TIVAS arm. However, there is much uncertainty associated with the results.
Conclusions:
Compared with Hickman catheters, TIVAS may be the cost-effective option. A larger multicentre trial is needed to confirm these preliminary findings.
Similar content being viewed by others
Main
When intravenous chemotherapy is needed it can either be given through a peripheral cannula (typically in a forearm vein) or through a central venous access device where the catheter tip is placed in a large central vein (typically the superior vena cava). Peripheral administration of chemotherapy frequently causes local vein irritation and thrombosis. This results in rapid exhaustion of the forearm veins, interruption to treatment, patient discomfort and a genuine fear of cannulation (Cheung et al, 2009). When the catheter tip lies centrally in a large vein, the damage is mitigated due to rapid blood flow and large vessel diameter. These advantages make central devices the obvious choice for longer drug regimes.
There are three main types of central device: (i) tunnelled central catheter commonly referred to as a Hickman; (ii) peripherally inserted central catheter (PICC); and (iii) totally implanted venous access system (TIVAS) commonly referred to as a port (Bishop et al, 2007). A recent informal survey (personal communications) of nine large UK cancer units indicated Hickman (58%) to be the most common followed by PICC (33%), with TIVAS only used in 9%. The TIVASs are more expensive, more complex and invasive to insert, and many healthcare staffs are unfamiliar with their aftercare. However, there is some evidence that TIVAS may have the lower complication rate and lead to greater patient satisfaction with less interruption to treatment regimens (Kulkarni et al, 2014). The evidence is weak and the studies are heterogeneous, in terms of patient populations, methodological approach and definition of outcomes. Therefore, the magnitude of this reduced risk is still unclear.
There is a need to evaluate the value of these devices to the UK NHS by looking at clinical and cost-effectiveness. It is unclear whether the higher purchasing costs of TIVAS may be offset by the perceived clinical benefits of lower complication rates and greater patient satisfaction. This phase II pilot trial aimed to inform the design of a larger definitive randomised controlled trial (RCT) by generating information about potential recruitment rates, incidence and distribution of outcome events, and the potential cost-effectiveness of the devices.
Materials and Methods
Study design and participants
This study was a phase II prospective, randomised, open trial conducted at two regional oncology centres in Scotland. All oncology patients with solid tumours, aged 18 years and over, who required a central venous access device for the delivery of chemotherapy, were eligible to participate in the study. Those who had evidence of any medical or psychiatric disorders that would be a contraindication to study participation and those with life expectancy of <3 months were excluded. This trial was reviewed and approved by the Multicentre Research Ethics Committee (11/AL/0083).
Randomisation and masking
All eligible patients were centrally randomised using minimisation, with respect to body mass index (BMI; <20, 20 to <30, 30 to <40, ⩾40), with a random element. A 3 : 1 (Hickman : TIVAS) randomisation ratio was used because of the limited availability and the cost of TIVAS. It was not feasible to mask participants and nurses to the allocated treatment.
Procedures
All devices were placed at one site under local anaesthesia with the patient option of conscious sedation. Hickman catheters were either single or double lumen; TIVASs were single lumen devices. The majority of the devices were placed by senior interventional radiologists, with a small number of Hickman catheters placed by a nurse-led venous access team. All devices were placed using jugular veins for access with ultrasound guidance. The positioning of the Hickman catheters was confirmed by fluoroscopy or chest X-ray; fluoroscopy was routinely used to position the TIVAS. A standardised approach to catheter care was adopted, which included weekly heparin flush and dressing change for the Hickman catheters, and monthly heparin flush for TIVAS. Unlike the Hickman catheters, TIVASs were not in routine use at either of the two centres before the study. Therefore, chemotherapy nursing staff received training before the start and during the study to minimise the potential impact of the ‘learning curve’.
Outcomes
The primary end point was overall complication rate. Complications included infection (blood stream infection, wound or exit site infection) and mechanical complications (line occlusion, migration, accidental withdrawal, flipping, central venous thrombosis, wound haematoma and skin breakdown or ulceration). Secondary end points included incidences of individual complications, time to first complication, health-related quality of life and resource use. Time to first complication was defined as the time from study registration until confirmed complication. Patients who did not experience a complication were censored at the date of device removal, date of last chemotherapy if the device had not been removed, the date of withdrawal if the patient withdrew from the study before experiencing complications or date of death. Health-related quality of life was assessed using a specifically designed 16-question device-specific questionnaire (Supplementary Appendix I) and the EuroQoL 5D (EQ-5D). The EQ-5D was recorded at baseline and monthly thereafter until device removal, death or end of follow-up. Resource use was recorded as consultations with healthcare professionals (inpatient stay, outpatient visits and general practitioner consultations). Patients were recruited between August 2011 and July 2013; the 12-month follow-up was completed in July 2014.
Statistical analysis
The sample size calculation was based on a randomised phase II screening approach to provide initial evidence of the effect of TIVAS in lowering the complication rate relative to Hickman catheters (Rubinstein et al, 2005). Only one UK study had previously compared Hickman and TIVAS-associated complications in patients undergoing chemotherapy (Ng et al, 2007), and reported a complication rate of ∼60% with Hickman catheters. The current phase II trial was designed to have 82% power to produce a statistically significant result at the 20% one-sided level of statistical significance if the true complication rate with TIVAS is 40%. This corresponds to an odds ratio (OR) of 2.25, which is at the low end of the estimates obtained from the wider literature (Carde et al, 1989; Kappers-Klunne et al, 1989; Mueller et al, 1992; Dillon et al, 2004; Johansson et al, 2004). The intention was to randomise 75 patients to Hickman catheters and 25 patients to TIVAS.
All analyses were performed on the intention-to-treat principle. Logistic regression was used for the primary analysis to compare the proportion of patients on each arm experiencing one or more complication; the model included the stratification variable used in the randomisation (BMI). Time to first complication was analysed as a secondary end point using a Cox regression, also including BMI in the model. Quality of life analysis was based on the device-specific questionnaire. Overall, 16 questions were graded on a scale of 1 (not at all) to 4 (very much). The worst score reported during the study was established for each question and these were compared across study arms via Mann–Whitney U-tests. The P-values for the individual questions were adjusted for multiple comparisons using the false-discovery rate approach (calculated using the p.adjust function of the stats library in R (http://www.r-project.org).
Pre-trial economic modelling
A probabilistic decision analytical model was used to evaluate the potential cost-effectiveness of Hickman catheters and TIVAS from the perspective of the UK NHS over the trial period (12 months). A simple decision tree structure was adopted to identify patients who may and may not experience complications. Data relating to complication rates, resource use, costs and health utilities were based on the results of the current phase II trial. The cost of Hickman catheters and TIVAS were costed at £80 and £300, respectively. The costs associated with the devices were calculated by applying unit costs to healthcare resource use. Health utilities and quality-adjusted life years (QALYs) were calculated from the EQ-5D data. Multiple imputation was used to impute missing values of the EQ-5D five dimensions (Rubin and Schenker, 1986), and mean QALYs were estimated using the area-under-curve approach (Dolan, 1997). Where appropriate, cost-effectiveness was expressed as incremental cost per complication averted and incremental cost per QALY gained. Probabilistic (via a 1000 iteration Monte Carlo simulation) and univariate sensitivity analyses were undertaken to assess uncertainty.
To examine whether conducting a larger RCT of Hickman lines versus TIVAS may be worthwhile, an expected value of perfect information (EVPI) analysis was carried out (Drummond et al, 2007). The analysis combined the probability and the cost of making the wrong decision, in terms of forgone health benefit and wasted resources based on uncertainty in the existing data. For the model, it was assumed that the life of technology is 5 years and the number of eligible patients per annum has been estimated at 425 000 per annum in the United Kingdom (HES data 2009–2010). A sample size calculation for a future trial was also undertaken based on the results of the economic evaluation using the net monetary benefit (NMB) approach (Supplementary Appendix II; Briggs, 2000). The estimates for both the cost and the effects were combined to determine the sample size for a cost-effectiveness outcome, using the traditional statistical methods for mean effectiveness, but based on the expected change in NMB (i.e., the change in monetarised effect minus the change in cost between the two alternatives; Briggs, 2000; Armitage et al, 2002).
Results
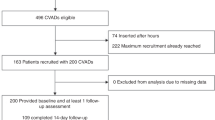
Seventy-four patients were randomised to Hickman catheters and 26 to TIVAS (Figure 1). One patient randomised to the TIVAS arm received a Hickman catheter due to administrative error. Three patients withdrew from the study before device insertion (two Hickman arm and one TIVAS arm). Devices were all successfully placed in 97 patients. The majority (Hickman 93% and TIVAS 84%) were inserted on the day of randomisation, and the remainder within 6 days. No immediate complications occurred during device placement. The two arms were well balanced for demographic and clinical baseline characteristics (Table 1). Colorectal, breast and pancreatic cancers made up the majority of the tumour types.
Complications
Forty (54%) Hickman patients reported one or more complication compared with 10 (38%) TIVAS patients (Table 2). On the basis of logistic regression model, taking into account BMI stratification, Hickman catheters were associated with a statistically significant increased risk (the threshold for statistical significance was based on the pre-defined statistical plan of this phase II study) of one or more complications compared with TIVAS devices (OR 2.07; 80% CI 1.11, 3.88; exact one-sided P=0.068).
There were 28 blood stream infections in total, 27 in 20 Hickman patients and in 1 TIVAS patient. Blood stream infection was the commonest complication in the Hickman arm, accounting for 45% of the complications. Fifteen patients, all in the Hickman arm required device removal due to blood stream infection. There were 30 line occlusions, 19 in 15 Hickman patients and 11 in 6 TIVAS patients. Line occlusion was the commonest complication in the TIVAS arm accounting for 55% of the complications. These were primarily resolved through simple catheter flushes and none required device removal in the TIVAS arm. In contrast, two patients in the Hickman arm required device removal due to occlusion. One patient in each arm had a confirmed central venous thrombosis; there were no reported pulmonary embolic events and no devices removed due to venous thrombosis. Overall, 21 devices were removed due to complications—20 from the Hickman arm and 1 from the TIVAS arm. In the Hickman arm, these were for infection (15), line occlusion (2), device malfunction (1), wound/exit site infection (1) and other (1); in the TIVAS arm, one single device was removed due to device malfunction. The median time to first complication for the Hickman arm was 30 weeks (80% CI 19, not estimable). The median time to first complication was not calculable for the TIVAS arm, as <50% of the patients experienced a complication.
Chemotherapy was interrupted due to complications in 12 patients in the Hickman arm and two in the TIVAS arm. In the Hickman arm, the duration of chemotherapy interruption ranged from 4 to 41 days, and in the TIVAS arm both interruptions were for 1 day only.
Quality of life
Overall, quality of life based on the device-specific questionnaire was better in TIVAS patients than Hickman patients. The adjusted one-sided P-values indicated that there were statistically significant differences at the 20% level in favour of TIVAS for all but three of the questions relating to ‘getting in and out of a car’, ‘using public transport’ and ‘going out shopping’ (Table 3).
Cost-effectiveness
In consequence to the higher complications rate, patients in the Hickman arm incurred significantly greater healthcare resource use than the TIVAS arm (Supplementary Appendix III). The health utilities fluctuated over the 12-month period in both the arms. In base-case analysis, Hickman catheters were associated with substantially greater mean cost (£2515 vs £712), fewer complications averted (62 vs 46, based on a cohort of 100 patients) and lower mean QALYs than TIVAS over a 1-year period (Table 4). However, the observed difference in QALYs between the devices is extremely small (0.64 vs 0.65). Overall, the Hickman arm was associated with greater costs and lower health benefits, suggesting that TIVAS is the dominant strategy.
Univariate sensitivity analysis was undertaken to examine the impact of complication rates by adopting the data from the wider literature. The probabilities of complications were estimated from pooling the results from the current phase II trial with two existing RCTs using on a random effects model (Carde et al, 1989; Kappers-Klunne et al, 1989). The estimated pooled OR for any complications was 3.05 (95% CI 1.08, 8.64), this was used in the analysis. The difference in cost between Hickman catheters and TIVAS increased, but the impact on the QALYs was remained extremely small (Table 4). The healthcare resource use among patients in the TIVAS arm was extremely low in the current phase II trial, this was also tested in the sensitivity analysis. The mean cost of patient with complications was assumed to be the same in both arms, this has little impact on the overall results. However, the model is most sensitive to the health utility estimates. When the QALY estimates for the Hickman arm was increased by 20%, and when all health utilities estimates were adjusted for censoring, TIVAS was no longer the dominant strategy. The results of the probabilistic sensitivity analysis following 1000 replications of the model are presented on the cost-effectiveness plane (Figure 2). The majority of the point estimates suggest that Hickman catheters were associated with greater costs than TIVAS, but there is substantial uncertainty in the difference in QALYs between the two devices. The value of information analysis suggested that, given current decision uncertainty, and at a willingness to pay threshold of £20 000, additional research is potentially worthwhile if future research costs less than £42 million.
On the basis of the base case, a sample of 507 per arm will be sufficient to show a positive NMB in favour of TIVAS, given the likely improvement in QALYs, rate of complications and potential cost savings compared with Hickman. However, when taking into account the additional evidence from existing literature using the pooled OR for any complications, the estimated NMA becomes greater in favour of TIVAS, the resultant required sample per arm was lower (323 per arm).
Discussion
This pilot study found that Hickman catheters were associated with significantly greater risk of complications than ports (OR 2.07; 80% CI 1.11, 3.88). These findings are in line with the existing evidence (Kulkarni et al, 2014; Coady et al, 2015). The most commonly reported complication in the Hickman arm was blood stream infection. This is likely related to the external component of the device plus the need for more regular flushing (weekly). In contrast with a totally implanted device, only one case of infection was observed. In the TIVAS arm, the most commonly reported complication was line occlusion (defined as inability to aspirate blood). The decision analytical model showed that, despite the lower device costs, taking into account complications, Hickman catheters were associated with greater costs, fewer complications averted, but similar QALYs compared with TIVAS. The TIVAS is the dominant strategy and is the cost-effective option. However, the estimates were associated with substantial uncertainty, and the findings were highly sensitive to health utility estimates.
The expected costs of uncertainty can be interpreted as the EVPI, based on the assumption that perfect information can eliminate the possibility of adopting the wrong decision. This also represents the maximum that the healthcare system should be willing to pay for additional evidence to inform this decision in the future through further research. At a cost-effectiveness threshold of £20 000 per QALY, based on the assumption that 425 000 patients may be eligible for venous catheters in the United Kingdom per year and a conservative expected lifetime of 1 year for the catheter, the EPVI for the effective population is approximately £42 million. This represents the maximum that the healthcare system should be willing to pay for additional evidence to inform this decision in the future.
This pilot trial was designed to generate information about potential recruitment rates, incidence of distribution of outcome events and the potential cost-effectiveness, to inform the design of a larger definitive RCT by. In terms of recruitment, the recruitment rate was poor at the initial 12 months. However, this was resolved by introducing dedicated staff to act as ‘trial champion’. The champion interacted with the patient pathway at all the important stages and successfully engaged with both healthcare staff and patients. In term of assessing complication rates, the definitions of complications were clear, but further refinements to the definitions of mechanical complications and line occlusions would ensure more accurate classification and coding. For instance, line occlusion was the most frequently observed complications among patients with TIVAS. Further investigations found that on several occasions when nursing staff was not able to aspirate blood return, this was resolved by the medical staff successfully re-sitting the needle into the TIVAS. It is likely that several of these were misclassified as apparent ‘line occlusions’. Training is important with both these devices to minimise complications. At the start of this trial, a TIVAS user-training programme was instituted as these devices were not in regular use. Training and nurse confidence improved over the study period. This could be a potential confounder in future trials.
There were also limitations to the economic evaluation. Healthcare resource use recorded in the TIVAS arm was surprisingly low, especially when compared with the Hickman arm. This may reflect potential performance bias; the two senior radiologists who were responsible for insertion of the TIVAS were often involved in resolving TIVAS complications. As a result, the costs associated with TIVAS may have been underestimated. On the other hand, the EuroQol 5D was used to estimate health utilities associated with using the two devices, and showed very small differences between the two arms. This may be explained by the results being dominated by the toxicity of the chemotherapy and disease status. The device-specific quality of life questionnaire in contrast appeared sensitive to differences between the two devices with 13 of the 16 questions showing statistically significant differences. The QALYs associated with TIVAS may have been underestimated. Due to the small sample size, correlation between the two questionnaires was not explored. The uncertainty associated with the QALY estimates was an important driver to the EVPI results. There is a clear need for more accurate estimates of QALYs, which supports the conclusion that further research to reduce overall uncertainty is worthwhile.
This study suggests that the most expensive and least used device (TIVAS) may in fact be the most cost-effective. If confirmed with a larger trial, TIVAS could become the dominant strategy. This will require a programme of both training and education across the United Kingdom where currently TIVAS are only used in a highly selective manner and almost exclusively placed by medical staff.
A much larger multicentre trial is needed that should also include PICC to establish clinical and cost-effectiveness. The NIHR (HTA) has recently funded a large RCT comparing Hickman lines, TIVAS and PICC (HTA 11/67/01). This trial (CAVA) of up to 2000 subjects, based on the sample size calculation that took into account the data from this phase II study and the wider literature, is currently underway.
Conclusions
Cancer is a leading cause of death and many patients are treated with chemotherapy. Intravenous chemotherapy often necessitates a long-term venous access device. This pilot study provided preliminary evidence of a lower complication rate with TIVAS compared with Hickman catheters in patients receiving chemotherapy. This difference resulted in the Hickman arm being associated with greater costs and lower health benefits than the TIVAS arm and hence being less cost-effective. These preliminary findings need confirmation from a larger multicentre phase III trial that should also include PICCs, which are currently the most common device used for chemotherapy delivery in the United Kingdom.
Change history
26 April 2016
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Armitage P, Berry G, Matthews JNS (2002) Analysing means and proportions. Blackwell Publishing: Oxford, London.
Bishop L, Dougherty L, Bodenham A, Mansi J, Crowe P, Kibbler C, Shannon M, Treleaven J (2007) Guidelines on the insertion and management of central venous access devices in adults. Int J Lab Hematol 29: 261–278.
Briggs A (2000) Economic evaluation and clinical trials: size matters. BMJ 321: 1362–1363.
Carde P, Cosset-Delaigue MF, Laplanche A, Chareau I (1989) Classical external indwelling central venous catheter versus totally implanted venous access systems for chemotherapy administration: a randomized trial in 100 patients with solid tumors. Eur J Cancer Clin Oncol 25: 939–944.
Cheung E, Baerlocher MO, Asch M, Myers A (2009) Venous access: a practical review for 2009. Can Fam Physician 55: 494–496.
Coady K, Ali M, Sidloff D, Kenningham RR, Ahmed S (2015) A comparison of infections and complications in central venous catheters in adults with solid tumours. J Vasc Access 16: 38–41.
Dillon PW, Jones GR, Bagnall-Reeb HA, Buckley JD, Wiener ES, Haase GM Children's Oncology G. (2004) Prophylactic urokinase in the management of long-term venous access devices in children: a Children's Oncology Group study. J Clin Oncol 22: 2718–2723.
Dolan P (1997) Modeling valuations for EuroQol health states. Med Care 35: 1095–1108.
Drummond MF, Sculpher MJ, Torrance GW, O'Brien BJ, Stoddart GL (2007) Methods for the Economic Evaluation of Health Care Programmes. Oxford University Press: Oxford, London.
Gray A, Clarke PM, Wolstenholme JL, Wordsworth S (2011) ASKEWS & Holts Library Services. Applied Methods of Cost-Effectiveness Analysis in Healthcare. Oxford University Press: Oxford, London.
Johansson E, Bjorkholm M, Bjorvell H, Hast R, Takolander R, Olofsson P, Backman L, Weitzberg E, Engervall P (2004) Totally implantable subcutaneous port system versus central venous catheter placed before induction chemotherapy in patients with acute leukaemia-a randomized study. Support Care Cancer 12: 99–105.
Kappers-Klunne MC, Degener JE, Stijnen T, Abels J (1989) Complications from long-term indwelling central venous catheters in hematologic patients with special reference to infection. Cancer 64: 1747–1752.
Kulkarni S, Wu O, Kasthuri R, Moss JG (2014) Centrally inserted external catheters and totally implantable ports for the delivery of chemotherapy: a systematic review and meta-analysis of device-related complications. Cardiovasc Intervent Radiol 37: 990–1008.
Mueller BU, Skelton J, Callender DP, Marshall D, Gress J, Longo D, Norton J, Rubin M, Venzon D, Pizzo PA (1992) A prospective randomized trial comparing the infectious and noninfectious complications of an externalized catheter versus a subcutaneously implanted device in cancer patients. J Clin Oncol 10: 1943–1948.
Ng F, Mastoroudes H, Paul E, Davies N, Tibballs J, Hochhauser D, Mayer A, Begent R, Meyer T (2007) A comparison of Hickman line- and Port-a-Cath-associated complications in patients with solid tumours undergoing chemotherapy. Clin Oncol (R Coll Radiol) 19: 551–556.
Rubin DB, Schenker N (1986) Multiple imputation for interval estimation from simple random samples with ignorable nonresponse. J Am Stat Assoc 81: 366–374.
Rubinstein LV, Korn EL, Freidlin B, Hunsberger S, Ivy SP, Smith MA (2005) Design issues of randomized phase II trials and a proposal for phase II screening trials. J Clin Oncol 23: 7199–7206.
Acknowledgements
The study was funded by a research grant from the Chief Scientist Office Scotland (CZG/2/512) and a small grant from Cook Medical UK. The funders had no role in the design of the study, data collection, data analysis, data interpretation or the writing of the paper. All authors assume responsibility for the accuracy and completeness of the data, and for the overall integrity of the article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Wu, O., Boyd, K., Paul, J. et al. Hickman catheter and implantable port devices for the delivery of chemotherapy: a phase II randomised controlled trial and economic evaluation. Br J Cancer 114, 979–985 (2016). https://doi.org/10.1038/bjc.2016.76
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2016.76
Keywords
This article is cited by
-
Cost-utility analysis of different venous access devices in breast cancer patients: a decision-based analysis model
BMC Health Services Research (2023)
-
Unexpected tunnelled central venous access demise: a single institutional study from the UK
Pediatric Surgery International (2021)
-
The risk of bloodstream infection associated with totally implantable venous access ports in cancer patient: a systematic review and meta-analysis
Supportive Care in Cancer (2020)