Abstract
To compare the efficacy of peripherally inserted central catheters (PICCs) and totally implantable venous-access ports (TIVAPs) for chemotherapy of pediatric patients with malignant tumors. A total of 96 children with malignant tumors who received catheterization of PICCs or TIVAPs for chemotherapy from May 2020 to May 2021 in Department of Pediatric Oncology of Qilu Hospital of Shandong University were selected. Then, the pathological features of disease, the age of children, the indwelling time, the incidence of postoperative complications, and the satisfaction degree were compared between the two groups. The age of children in the TIVAP group was younger than that in the PICC group (P < 0.05). The indwelling time in the TIVAP group was 7.2 ± 2.757 months,which was significantly longer than 5.65 ± 2.058 months in the PICC group (P < 0.05). The incidence of postoperative complications in the TIVAP group without systemic or local infection was markedly lower than that in the PICC group (P < 0.05). The satisfaction degree of patients in the TIVAP group without unsatisfied was markedly higher than that in the PICC group (P < 0.05). TIVAPs may be the first choice for chemotherapy of children with malignant tumors.
Similar content being viewed by others
Introduction
The incidence of childhood cancer has increased over the past few decades worldwide1,2,3,4,5,6. Cancer is the second most common cause of death in children who were aged 0–14 years old. The most common types of childhood cancers include leukemia, brain cancer, lymphoma, and other solid tumors6. Although the overall survival rate of children with acute lymphoblastic leukemia (ALL) is more than 90%, leukemia remains one of the leading causes of death7. Fortunately, increasing knowledge on biology and tumorigenesis of childhood cancer contributes to advance in chemotherapy, supportive care, and personalized medicine7. However, cancers, especially childhood cancers, are currently treated by chemotherapy, and a long-term venous access is essential for the children with malignant diseases.
Central venous catheters (CVCs) reduce the stimulation to the vein and skin and improve injection of liquid medicines, which are usually used for patients receiving chemotherapeutic drugs8. Both peripherally inserted central catheters (PICCs) and totally implantable venous-access ports (TIVAPs) are frequently used for chemotherapy of children with cancer9. It has been shown that PICCs possess various advantages, such as convenient placement without pleura-pulmonary damage, low cost, and long-term and stable vein access10. At present, PICCs are extensively utilized in clinical management of chemotherapy, medication administration, and parenteral nutrition. For pediatric patients, due to vascular conditions, their own distress, non-cooperation and other reasons, the application of PICCs is limited, however, TIVAPs are a compensation for its deficiency. TIVAPs assist patients with a safe and permanent access to a vein, which are often used in patients who need continuous administration of intravenous drugs, including those receiving chemotherapy12. TIVAPs are also used for the purposes of regular intravenous medications, transfusion of blood products, parenteral nutrition, or the necessity of regular periodic blood sampling13. This retrospective study aimed to compare the efficacy of PICCs and TIVAPs for chemotherapy of pediatric patients with malignant tumors.
Results
Childhood retrospective cohort: a case–control study of childhood cancers treated with PICCs or TIVAPs
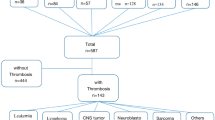
An overview of the study is shown in Fig. 1. 96 childhood patients with cancers who were treated with PICCs or TIVAPs from May 2020 to May 2021 were retrospectively analyzed. A timeline of the study is shown in Fig. 1.
Patients’ demographic and clinical data
Patients’ age in the TIVAP group was significantly younger than that in the PICC group (P < 0.05). The frequency of age in two groups is shown in Fig. 2. There was no significant difference between the two groups in terms of gender and pathological features of the disease (P > 0.05) (Table 1).
The indwelling time
The indwelling time was 7.2 ± 2.757 months in the TIVAP group, which was significantly longer than 5.65 ± 2.058 months in the PICC group (P < 0.05) (Fig. 3).
Postoperative complications
In the PICC group, there were 21 cases with local infection, 11 with phlebosclerosis, 11 with local allergy, 3 with local bleeding, 1 with thrombogenesis, 1 with catheter displacement, and 9 with catheter obstruction. In the TIVAP group, there were only 9 cases with catheter obstruction. The incidence of postoperative complications including local infection, phlebosclerosis, and local allergy in the TIVAP group was markedly lower than that in the PICC group (P < 0.05) (Table 2). Moreover, with any complications as dependent variables and gender, age, pathological features, indwelling time as independent variables, logistic regression was performed. It was found that compared with PICC group, TIVAP group had a lower incidence of complications, with OR = 0.000 (95%CI 0.000–0.132), and long indwelling time may be a risk factor of complications (P = 0.021) after adjusting the factors of gender, age, pathological type and indwelling time (Table 3).
Catheter-related satisfaction
Satisfaction questionnaire was implemented in the two groups. In the PICC group, 38 cases were highly satisfied, 9 were moderately satisfied, and 1 was not satisfied. In the TIVAP group, 46 cases were satisfied, of whom 1 case was moderately satisfied. No patient was found unsatisfied in the TIVAP group. All questionnaires without complications were "satisfied", so the impact of complications on satisfaction was not considered in the multivariate regression analysis. With satisfaction (Satisfied = 1, basically satisfied or not satisfied = 2) as dependent variables and gender, age, pathological features, indwelling time as independent variables, logistic regression was performed, and it was found that the satisfaction degree of children in the TIVAP group was significantly higher than that in the PICC group OR = 0.119 (95%CI 0.018–0.791) after adjusting the factors of gender, age, pathological type, indwelling time and complications (Table 3).
Discussion
As childhood malignant tumors that have an increasing incidence rate, a high recurrence rate or high persistence but a low mortality rate, leukemia or other solid cancers have different histological types and subtypes, as well as cell sources, characteristics and prognoses. Chemotherapy and laboratory tests of blood are necessary for these childhood patients. CVCs allow safe administration of intravenous cytotoxic medications, facilitate the administration of intravenous fluid resuscitation or parenteral nutrition, and help blood collection for relieving the pain. CVCs have been largely used in oncology department in China14. In addition, CVCs can protect peripheral veins and support chemotherapy with significant advantages compared with peripheral vein catheters15. In the present study, all CVCs were used for chemotherapy. This study compared the efficacy of TIVAPs and PICCs for chemotherapy of children with cancer.
TIVAPs not only assist children with a safe vascular access for treatment, but also protect vessels from repeated puncture injuries16. According to our experience, in children, the younger the patients are, the greater the advantages of TIVAPs will be. The distance from PICC to the venous tube is slightly longer, which may increase the risk of traction and infection due to children's activities. The catheter is observable out of the body, children’s clothing is partially limited, the exposed catheter is easy to prolapse or to be accidentally pulled out which is associated with catheter displacement, and it may also be accompanied by leakage during activities or hemorrhage. However, an implantable port is fully implanted subcutaneously without any exposed part and cannot be removed by itself. Besides, the distance to the tube is shorter than that of PICC, thus, the risk of infection is reduced17. Patel et al.9 and Taxbro et al.11 reported a lower incidence of postoperative complications for TIVAPs compared with PICCs in randomized controlled trials. Therefore, it is appropriate for children with hyperactivity and a strong sense of curiosity. Although not statistically significant, there seems a difference in the cancer types between TIVAP and PICC, which may be explained by the treatment cycles of the cancer types. The longer the treatment period, the more likely TIVAP because of its long lifespan.
In the current study, the longest indwelling time was 7.2 ± 2.757 months in the TIVAP group, while that was 5.65 ± 2.058 months in the PICC group. According to the guidelines, the indwelling time in PICC group was 12 months, while that was 240 months in the TIVAP group (in theory). However, in our ward, the indwelling time in the PICC group was about 8 months, and no TIVAP was taken out from patients. For the treatment of children with ALL, the total course of disease is about 30 months, while that is within 12 months in the majority of cases with solid tumors. Thus, patients requiring long-term treatment are better candidates for TIVAPs, which is consistent with the previous findings16.
TIVPA was found to be associated with postoperative complications, and it was related to a higher satisfaction degree of patients compared to PICC in the present study, which is in agreement with the result of another research18,19. While CVCs are clinically popular, their complications, especially the serious complication of infections are still common and considered to cause damage to patients, which are associated with morbidity, mortality, and financial costs of the patients. At present, the prevention of CVCs-related injuries served by multidisciplinary team involvement is taken into consideration for healthcare researchers, clinicians and patients. Local infection is manifested as redness, swelling, heat and pain at the puncture site, while local allergy is manifested as red rashes with itchy sensation. In the present study, there were more local infections and local allergies in the PICCs group, which may be related to the local application of the plastic sticking dressings. Phlebophlogosis with or without thrombogenesis, a relatively common peripheral vascular disease, is aseptic inflammation of the veins. Standard practice requires PICC catheter insertion in the basilic vein, which is 0.4–0.5 cm in diameter compared to TIVAP catheter insertion into the subclavian vein, which is 1–2 cm in diameter. Although the lumens of both catheters are similar, the smaller diameter of the basilic veins used for the PICC catheter is likely to result in slower blood flow rates, increasing the risk of blood clot formation. On the other hand, the longer intravenous length of a PICC catheter increases the surface area for the propagation of thrombosis and catheter obstruction, while the TIVAP catheter tip without an anti-reflux device may explain the occurrence of catheter obstruction.
The major limitation of this study was the lack of cost analysis in the two groups. In some practice settings, TIVAP are currently the standard CVC in oncology. In our practice location, a 50–50 split between TIVAP and PICC may be related to cost or the risk of surgical procedures. There were direct and indirect costs, and direct costs included medical and non-medical costs. Medical costs covered costs related to drugs, surgery, laboratory tests, non-laboratory tests, and medical consumptive costs (e.g., a hospital’s expenses). Non-medical expenses included costs related to transportation, accommodation, and nutrition of children and their staffs. An indirect economic burden included the economic loss caused by the loss of working opportunities20. A study showed that there was no significant difference in medical expenses if a CVC would be still in place at 6 months after insertion9. Another study indicated that when the period of treatment was longer than 12 months, there was no significant difference in medical costs between TIVAP and PICC groups21. Thus, the daily expenses for CVCs were highly associated with the indwelling time, which was significantly longer in the TIVAP group. Another limitation of this study was non-randomized nature and inadequate sample size, which was mainly due to the limited number of patients at a single center. An inadequate sample size may have led to the lack of statistical significance in the time to the first major complication observed. Prospective randomized controlled studies with large sample size are required to further verify our findings.
Conclusions
In summary, TIVAPs were associated with a longer indwelling time and a lower incidence of postoperative complications, thereby confirming their higher efficiency compared with PICCs. TIVAPs are recommended for children with malignant tumors, in particular those requiring long-term treatment, and health-based educational programs are also recommended during implantation, so as to reduce the risk of postoperative complications and to improve the satisfaction rate of families of children with cancer. However, further prospective studies are required to validate our findings and to ascertain medical expenses and the incidence of postoperative complications, in order to better highlight the cost-effectiveness of CVCs.
Patients and methods
Subjects
Clinical data of 96 patients with cancer who were treated with PICCs or TIVAPs in Department of Pediatric Oncology of Qilu Hospital of Shandong University (Jinan, China) from May 2020 to May 2021 were retrospectively analyzed.
Statement
PICCs method was carried out in accordance with nursing practice standards for intravenous therapy. TIVAPs method was carried out in accordance with Expert consensus and Technical Operation Guide for Clinical Application of TIVAPs (2017 edition). Authors confirm that all experimental protocols were approved by Ethics Committee of Shandong University Qilu Hospital. The research involving human research participants have been performed in accordance with the Declaration of Helsinki.
Inclusion criteria
Patients who were diagnosed by bone marrow and peripheral hemocytological changes or clinicopathology; patients who were aged < 18 years old and had no history of mental disorders; patients who were conscious; patients with complete clinical data; signed written informed consent form by patients’ parents before the study.
Exclusion criteria
Patients with multiple organ failure; patients with a poor compliance.
Methods
The Seldinger technique was used in both groups 22. A detailed description of the methods of TIVAPs and PICCs can be found in the literature9,21.
Observational indices
The pathological features of disease, the age of children, the indwelling time, the incidence of postoperative complications, and the satisfaction degree were compared between the two groups. The pathological features of disease and the age of children were identified in the two groups. The indwelling time was calculated monthly. Until the time of the study, if the catheter was removed, the indwelling time was calculated; if the catheter was not removed, the indwelling time was calculated as the time from the catheter insertion date to the study date. The satisfaction degree was assessed through a questionnaire, which was sent to patients' parents three months after catheterization with anonymous responses covering purpose, maintenance, management of postoperative complications, and patients’ attitudes toward catheters and staffs’ perceptions. The survey question 9,10 and 12 provided the data reported. The remaining questions were used to evaluate the management of maintenance. The designed questionnaire is presented in Supplementary information file.
Statistical analysis
The SPSS 21.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA) software were used to perform statistical analysis and graphical illustration, respectively. Categorical variables were expressed as percentages, and Chi-square test was used to compare data between two groups. Measured data were expressed as mean ± standard deviation (SD). P < 0.05 was considered statistically significant.
References
Baade, P. D. et al. Trends in incidence of childhood cancer in Australia, 1983–2006. Br. J. Cancer 102, 620–626 (2010).
Paapsi, K. et al. Childhood cancer incidence and survival trends in Estonia (1970–2016): A nationwide population-based study. BMC Cancer 20, 30 (2020).
Grabas, M. R. et al. Incidence and time trends of childhood cancer in Denmark, 1943–2014. Acta Oncol. 59, 588–595 (2020).
Linabery, A. M. & Ross, J. A. Trends in childhood cancer incidence in the U.S. (1992–2004). Cancer 112, 416–432 (2008).
Steliarova-Foucher, E. et al. Changing geographical patterns and trends in cancer incidence in children and adolescents in Europe, 1991–2010 (Automated Childhood Cancer Information System): A population-based study. Lancet Oncol. 19, 1159–1169 (2018).
Yang, L., Yuan, Y., Sun, T., Li, H. & Wang, N. Characteristics and trends in incidence of childhood cancer in Beijing, China, 2000–2009. Chin. J. Cancer Res. 26, 285–292 (2014).
Allemani, C. et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 391, 1023–1075 (2018).
Bertoglio, S., Faccini, B., Lalli, L., Cafiero, F. & Bruzzi, P. Peripherally inserted central catheters (PICCs) in cancer patients under chemotherapy: A prospective study on the incidence of complications and overall failures. J. Surg. Oncol. 113, 708–714 (2016).
Patel, G. S. et al. Comparison of peripherally inserted central venous catheters (PICC) versus subcutaneously implanted port-chamber catheters by complication and cost for patients receiving chemotherapy for non-haematological malignancies. Support Care Cancer 22, 121–128 (2014).
Mielke, D., Wittig, A. & Teichgraber, U. Peripherally inserted central venous catheter (PICC) in outpatient and inpatient oncological treatment. Support Care Cancer 28, 4753–4760 (2020).
Taxbro, K. et al. Clinical impact of peripherally inserted central catheters vs implanted port catheters in patients with cancer: An open-label, randomised, two-centre trial. Br. J. Anaesth 122, 734–741 (2019).
Tabatabaie, O. et al. Totally implantable venous access devices: A review of complications and management strategies. Am. J. Clin. Oncol. 40, 94–105 (2017).
Zhang, P. et al. Utility of totally implantable venous access ports in patients with breast cancer. Breast J. 26, 333–334 (2020).
Cai, Y., Zhu, M., Sun, W., Cao, X. & Wu, H. Study on the cost attributable to central venous catheter-related bloodstream infection and its influencing factors in a tertiary hospital in China. Health Qual. Life Outcomes 16, 198 (2018).
Böll, B. et al. Central venous catheter-related infections in hematology and oncology: 2020 updated guidelines on diagnosis, management, and prevention by the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Medical Oncology (DGHO). Ann. Hematol. 100, 239–259 (2021).
Han, L. et al. Totally implantable venous access ports: A prospective randomized study comparing subclavian and internal jugular vein punctures in children. J. Pediatr. Surg. 56, 317–323 (2021).
Pinelli, F. et al. Infection of totally implantable venous access devices: A review of the literature. J. Vasc. Access 19, 230–242 (2018).
Blanco-Guzman, M. O. Implanted vascular access device options: A focused review on safety and outcomes. Transfusion 58(Suppl 1), 558–568 (2018).
Gurkan, S. et al. Retrospective evaluation of totally implantable venous access port devices: Early and late complications. J. B.U.ON. Off. J. Balkan Union Oncol. 20, 338–345 (2015).
Zhou, Y. et al. Economic burden for retinoblastoma patients in China. J. Med. Econ. 23, 1553–1557 (2020).
Fang, S., Yang, J., Song, L., Jiang, Y. & Liu, Y. Comparison of three types of central venous catheters in patients with malignant tumor receiving chemotherapy. Patient Prefer. Adherence 11, 1197–1204 (2017).
Zou, Y. et al. Assessment of complications and short-term outcomes of percutaneous peritoneal dialysis catheter insertion by conventional or modified Seldinger technique. Ren. Fail. 43, 919–925 (2021).
Acknowledgements
This work was supported by Natural science fund of Shandong Province (ZR2020QH213) and Key Research and Development program of Shandong Province (2019GSF108060). Thanks to Si Libo and Zhang Aijun for the funding.
Author information
Authors and Affiliations
Contributions
Z.H.: Dr. Zhang conceptualized and designed the study, drafted the initial manuscript, and approved the final manuscript as submitted. Y.L.: Dr. Li carried out the initial analyses, reviewed and revised the manuscript, and approved the final manuscript as submitted. N.Z.: Dr. Zhu analysed the data and revised the manuscript, and approved the final manuscript as submitted. Y.L.: Dr. Li analysed the data and revised the manuscript, and approved the final manuscript as submitted. J.F.: Prof. Fu designed the data collection instruments, and coordinated and supervised data collection, critically reviewed the manuscript, and approved the final manuscript as submitted. J.L.: Prof. Liu performed statistical analyses, and approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, H., Li, Y., Zhu, N. et al. Comparison of peripherally inserted central catheters (PICCs) versus totally implantable venous-access ports in pediatric oncology patients, a single center study. Sci Rep 12, 3510 (2022). https://doi.org/10.1038/s41598-022-07584-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-07584-8
This article is cited by
-
Exploring risk factors for totally implantable venous access devices (TIVADs)-related thrombotic occlusion in the off-treatment period
Scientific Reports (2023)
-
Peripherally inserted central catheters can be an alternative to tunneled central venous catheters in chemotherapy for hematological and oncological pediatric patients
Pediatric Surgery International (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.