Abstract
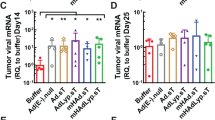
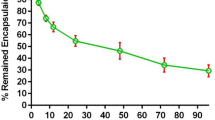
Gene therapy utilizing lipid-based delivery systems holds tremendous promise for the treatment of cancer. However, due to the potential adverse inflammatory and/or immune effects upon systemic administration, treatments thus far have been predominantly limited to intratumoral or regional treatment. Previous studies from our group have demonstrated the antitumor efficacy of systemically administered, folate-targeted, lipid–protamine–DNA complexes (LPD-PEG-Folate) against breast cancer using an immunodeficient xenogenic murine model. In the current study, the antitumor efficacy of LPD-PEG-Folate in a syngeneic, immune competent, murine model of breast cancer was examined. In this model, the potential inflammatory or immune responses and their effects on systemic delivery can be addressed. The 410.4 murine breast adenocarcimona cell line was initially evaluated in vitro for its interactions with LPD-PEG-Folate and control LPD-PEG formulations. Utilizing fluorescently labeled formulations and fluorscence-activated cell sorting (FACS) analysis, a 1.6-fold enhancement of binding and internalization of LPD-PEG-Folate over LPD-PEG formulations was observed, suggestive of specific receptor interaction. Increased binding was manifested as 5–26-fold increases in luciferase gene expression in 410.4 cell transfection when comparing LPD-PEG-Folate to LPD-PEG. Moreover, in vivo treatment of 410.4 breast tumors in BALB/c mice with i.v. injected LPD-PEG-Folate delivering the HSV-1 thymidine kinase (TK) gene, in combination with gancyclovir treatment, resulted in a significant reduction in mean tumor volume (260.1 mm3) compared to the LPD-PEG-TK (914.1 mm3), as well as the vehicle (749.7 mm3) and untreated (825.3 mm3) control groups (day 25, P<.019). In addition to a reduced tumor volume, LPD-PEG-Folate-TK treatment also increased median survival from 25 days in the nontargeted LPD-PEG-TK groups to 31 days (P=.0011), which correlated with the termination of treatment. Together, these results demonstrate that in the context of a fully functional immune system, LPD-PEG-Folate-TK treatment possesses significant specific antitumor efficacy and the potential for further preclinical development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Abbreviations
- LPD:
-
lipid–protamine–DNA
- TK:
-
thymidine kinase
- HSV-1:
-
herpes simplex virus
- DOTAP:
-
1,2-dioleoyl-3-trimethylammonium-propane
- GCV:
-
ganciclovir
- DSPE-PEG5K:
-
1,2-disteraoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-5K]
- LPD-PEG:
-
DOTAP:CHOL:DSPE-PEG5K (12:1:1 nmol lipid: μg protamine: μg DNA ratio)
- LPD-PEG-Folate:
-
DOTAP:CHOL:DSPE-PEG5K-Folate (12:1:1 nmol lipid: μg protamine: μg DNA ratio)
References
Ueno NT, Bartholomeusz C, Herrmann JL, et al. E1A-mediated paclitaxel sensitization in HER-2/neu-overexpressing ovarian cancer SKOV3.ip1 through apoptosis involving the caspase-3 pathway. Clin Cancer Res. 2000;6:250–259.
Yoo GH, Hung MC, Lopez-Berestein G, et al. Phase I trial of intratumoral liposome E1A gene therapy in patients with recurrent breast and head and neck cancer. Clin Cancer Res. 2001;7:1237–1245.
Jacobs A, Voges J, Reszka R, et al. Positron-emission tomography of vector-mediated gene expression in gene therapy for gliomas. Lancet. 2001;358:727–729.
Otsuka M, Baru M, Delriviere L, Talpe S, Nur I, Gianello P . In vivo liver-directed gene transfer in rats and pigs with large anionic multilamellar liposomes: routes of administration and effects of surgical manipulations on transfection efficiency. J Drug Target. 2000;8:267–279.
Li S, Huang L . In vivo gene transfer via intravenous administration of cationic lipid–protamine–DNA (LPD) complexes. Gene Ther. 1997;4:891–900.
Sorgi FL, Bhattacharya S, Huang L . Protamine sulfate enhances lipid-mediated gene transfer. Gene Ther. 1997;4:961–968.
Ueno NT, Bartholomeusz C, Xia W, et al. Systemic gene therapy in human xenograft tumor models by liposomal delivery of the E1A gene. Cancer Res. 2002;62:6712–6716.
Whitmore M, Li S, Huang L . LDP lipopolyplex initiates a potent cytokine response and inhibits tumor growth. Gene Ther. 1999;6:1867–1875.
Li S, Rizzo MA, Bhattacharya S, Huang L . Characterization of cationic lipid–protamine DNA (LPD) complexes for intravenous gene delivery. Gene Ther. 1998;5:930–937.
Tan Y, Zhanng JS, Huang L . Codelivery of NF-κB decoy-related oligodeoxynucleotide improves LPD-mediated systemic gene transfer. Mol Ther. 2002;6:1–9.
Shibata M-A, Morimoto J, Otsuki Y . Suppression of murine mammary carcinoma growth and metastases by HSVtk/GCV gene therapy using in vivo electroporation. Cancer Gene Ther. 2002;9:16–27.
Majumdar AS, Zolotorev A, Samuel S, et al. Efficacy of herpes simplex virus thymidine kinase in combination with cytokine gene therapy in an experimental metastatic breast cancer model. Cancer Gene Ther. 2000;7:1086–1099.
Link CJ, Levy JP, McCann LZ, Moorman DW . Gene therapy for colon cancer with herpes simplex thymidine kinase gene. J Surg Oncol. 1997;64:289–294.
Pantuck AJ, Matherly J, Zisman A, et al. Optimizing prostate cancer gene therapy using herpes simplex virus thymidine kinase active site variants. Human Gene Ther. 2002;13:777–789.
Sugaya S, Fujita K, Kikuchi A, et al. Inhibition of tumor growth by direct intratumoral gene transfer of herpes simplex virus thymidine kinase gene with DNA-liposome complexes. Human Gene Ther. 1996;7:223–230.
Princen F, Lechanteur C, Lopez M, Gielen J, Bours V, Merville M-P . Similar efficiency of DNA-liposome complexes and retrovirus-producing cells for HSV-tk suicide gene therapy of peritoneal carcinomatosis. J Drug Targeting. 2000;8:79–89.
Black ME . Enzyme and pathway engineering for suicide gene therapy. Genet Eng (NY). 2001;23:113–127.
Spencer DM . Developments in suicide genes for preclinical and clinical applications. Current Opin Mol Therap. 2000;2:433–440.
Freeman SM, Abboud CN, Whartenby KA, et al. The “bystander effect”: tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res. 1993;53:5274–5283.
Xu L, Tang W-H, Huang C-C, et al. Systemic p53 gene therapy of cancer with immunopolyplexes targeted by anti-transferrin receptor scFv. Mol Med. 2001;7:723–734.
Zitzmann S, Ehemann V, Schwab M . Arginine-glycine-aspartic acid (RGD)-peptide binds to both tumor and tumor-endothelial cells in vivo. Cancer Res. 2002;62:5139–5143.
Hood JD, Benarski M, Frausto R, et al. Tumor regression by targeted gene delivery to the neovasculature. Science. 2002;296:2404–2407.
Harvie P, Galbraith T, Dutzar B, et al. Folate targeted cationic lipid–protamine–DNA (LPD) complexes: in vitro and in vivo characterization. Mol Ther. 2002;5:59.
Lupton SD, Brunton LL, Kalberg VA, Overell RW . Dominant positive and negative selection using a hygromycin phosphotransferase-thymidine kinase fusion gene. Mol Cell Biol. 1991;11:3374–3378.
Yoo GH, Hung MC, Alexander W, et al. Systemic p53 gene therapy of cancer with immunolipoplexes targeted by anti-transferrin receptor scFv. Mol Med. 2001;7:723–734.
Dexter DL, Kowalski HM, Blazar BA, Fligiel Z, Vogel R, Heppner GH . Heterogeneity of tumor cells from a single mouse mammary tumor. Cancer Res. 1978;38:3174–3181.
Heppner GH, Dexter DL, DeNucci T, Miller FR, Calabresi P . Heterogeneity in drug sensitivity among tumor cell populations of a single mouse mammary tumor. Cancer Res. 1978;38:3758–3763.
Blazar BA, Laing CA, Miller FR, Heppner GH . Activity of lymphoid cells separated from mammary tumors in blastogenesis and Winn assays. J Natl Cancer Inst. 1980;65:405–410.
Harvie P, Bruckheimer E, Dutzar B, et al. Systemically deliverable targeted lipid–polycation–DNA (LPD) lipopolyplex for gene delivery in the treatment of cancer. Cancer Gene Ther. 2002;10:S25.
Miller FR, Miller BE, Heppner GH . Characterization of metastatic heterogeneity among subpopulations of a single mouse mammary tumor: heterogeneity in phenotypic stability. Invas Metast. 1983;3:22–31.
Claassen E . Post-formation fluorescent labeling of liposomal membranes. In vivo detection, localization, and kinetics. J Immunol Methods. 1992;147:231–240.
Acknowledgements
We greatly acknowledge Drs Ziv Sandalon and Edward Kelly for their constructive comments and review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bruckheimer, E., Harvie, P., Orthel, J. et al. In vivo efficacy of folate-targeted lipid–protamine–DNA (LPD-PEG-Folate) complexes in an immunocompetent syngeneic model for breast adenocarcinoma. Cancer Gene Ther 11, 128–134 (2004). https://doi.org/10.1038/sj.cgt.7700662
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7700662