Abstract
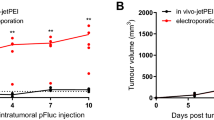
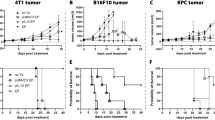
In this study, we measured transfection efficiency in vitro and in vivo using the following nonviral approaches of gene delivery: injection of plasmid DNA, electroporation-assisted, liposome-enhanced, and integrin-targeted gene delivery, as well as the combination of these methods. Four histologically different tumor models were transfected with a plasmid encoding the green fluorescent protein (GFP) (B16 mouse melanoma, P22 rat carcinosarcoma, SaF mouse sarcoma, and T24 human bladder carcinoma) using adherent cells, dense cell suspensions, and solid tumors. Emphasis was placed on different electroporation conditions to optimise the duration and amplitude of the electric pulses, as well as on different DNA concentrations for effective gene delivery. In addition, transfection efficiency was correlated with cell density of the tumors. The major in vivo findings were: (a) electroporation-assisted gene delivery with plasmid DNA, employing long electric pulses with low amplitude, yielded significantly better GFP expression than short electric pulses with high amplitude; (b) electroporation combined with liposome–DNA complexes yielded the highest percentage of transfected tumor area in B16F1 tumor (6%); (c) transfection efficiency of electroporation-assisted plasmid DNA delivery was dependent on tumor type; (d) integrin-targeted vector, alone or combined with electroporation, was largely ineffective. In conclusion, our results demonstrate that some nonviral methods of gene delivery are feasible and efficient in transfecting solid tumors. Therefore, this makes nonviral methods attractive for further development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Harvey BG, Worgall S, Ely S et al. Cellular immune responses of healthy individuals to intradermal administration of an E1–E3–adenovirus gene transfer vector Hum Gene Ther 1999 10: 2823–2837
Marshall E . Gene therapy death prompts review of adenovirus vector Science 1999 286: 2244–2245
Cotten M, Wagner E . Non-viral approaches to gene therapy Curr Opin Biotechnol 1993 4: 705–710
Kikuchi A, Aoki Y, Sugaya S et al. Development of novel cationic liposomes for efficient gene transfer into peritoneal disseminated tumor Hum Gene Ther 1999 10: 947–955
Curiel DT, Agarwal S, Wagner E et al. Adenovirus enhancement of transferrin–polylysine–mediated gene delivery Proc Natl Acad Sci USA 1991 88: 8850–8854
Wagner E, Plank C, Zatloukal K et al. Influenza virus hemagglutinin HA-2 N-terminal fusogenic peptides augment gene transfer by transferrin–polylysine–DNA complexes: toward a synthetic virus-like gene-transfer vehicle Proc Natl Acad Sci USA 1992 89: 7934–7938
Simoes S, Slepushkin V, Gaspar R et al. Gene delivery by negatively charged ternary complexes of DNA, cationic liposomes and transferrin or fusogenic peptides Gene Ther 1998 5: 955–964
Hart SL, Arancibia-Carcamo CV, Wolfert MA et al. Lipid-mediated enhancement of transfection by a nonviral integrin-targeting vector Hum Gene Ther 1998 9: 575–585
Dachs GU, Coralli C, Hart SL et al. Gene delivery to hypoxic cells in vitro Br J Cancer 2000 83: 662–667
Jenkins RG, Herrick SE, Meng QH et al. An integrin-targeted non-viral vector for pulmonary gene therapy Gene Ther 2000 7: 393–400
Mir LM, Glass LF, Sersa G et al. Effective treatment of cutaneous and subcutaneous malignant tumors by electrochemotherapy Br J Cancer 1998 77: 2336–2342
Sersa G, Stabuc B, Cemazar M et al. Electrochemotherapy with cisplatin: clinical experience in malignant melanoma patients Clin Cancer Res 2000 6: 863–867
Somiari S, Glasspool-Malone J, Drabick JJ et al. Theory and in vivo application of electroporative gene delivery Mol Ther 2000 2: 178–187
Mir LM, Bureau MF, Gehl J et al. High-efficiency gene transfer into skeletal muscle mediated by electric pulses Proc Natl Acad Sci USA 1999 96: 4262–4267
Heller L, Jaroszeski MJ, Coppola D et al. Electrically mediated plasmid DNA delivery to hepatocellular carcinomas in vivo Gene Ther 2000 7: 826–829
Tozer GM, Shaffi KM . Modification of tumour blood flow using the hypertensive agent, angiotensin II Br J Cancer 1993 67: 981–988
Hill SA, Collinrigde DR, Vojnovic B et al. Tumour radiosensitization by high-oxygen-content gases: influence of the carbon dioxide, content of the inspired gas on pO2, microcirculatory function and radiosensitivity Int J Radiat Oncol Biol Phys 1998 40: 943–951
Cemazar M, Milacic R, Miklavcic D et al. Intratumoral cisplatin administration in electrochemotherapy: antitumor effectiveness, sequence dependence and platinum content Anticancer Drugs 1998 9: 525–530
Miklavcic D, Beravs K, Semrov D et al. The importance of electric field distribution for effective in vivo electroporation of tissues Biophys J 1998 74: 2152–2158
Cemazar M, Miklavcic D, Vodovnik L et al. Improved therapeutic effect of electrochemotherapy by intratumoral drug administration and changing of electrode orientation for electropermeabilization on EAT tumor model in mice Radiol Oncol 1995 29: 121–127
Nabel GJ, Nabel EG, Jang Z et al. Direct gene transfer with DNA–liposome complexes in melanoma: expression, biologic activity, and lack of toxicity in humans Proc Natl Acad Sci USA 1993 90: 11307–11311
Larchian WA, Horiguchi J, Nair SK et al. Effectiveness of combines interleukin-2 and B7.1 vaccination strategy is dependent on the sequence and order: a liposome-mediated gene therapy treatment for bladder cancer Clin Cancer Res 2000 6: 2913–2920
Yoo GH, Hung MC, Lopez-Berenstein G et al. Phase I trial of intratumoral liposome EiA gene therapy in patients with recurrent breast and head and neck cancer Clin Cancer Res 2001 7: 1237–1245
Wells JM, Li LH, Sen A et al. Electroporation-enhanced gene delivery in mammary tumors Gene Ther 2000 7: 541–547
Heller R, Schultz J, Lucas ML et al. Intradermal delivery of interleukin-12 plasmid DNA by in vivo electroporation DNA Cell Biol 2001 20: 21–26
Heller L, Pottinger C, Jaroszeski MJ et al. In vivo electroporation of plasmids encoding GM-CSF or interleukin-2 into existing B16 melanomas combined with electrochemotherapy induces long-term antitumour immunity Melanoma Res 2000 10: 577–583
Niu G, Heller R, Catlett-Falcone R et al. Gene therapy with dominant-negative Stat3 suppresses growth of the murine melanoma B16 tumor in vivo Cancer Res 1999 59: 5059–5063
Yamashita YI, Shimada M, Hasegawa H et al. Electroporation-mediated interleukin-12 gene therapy for hepatocellular carcinoma in the mice model Cancer Res 2001 61: 1005–1012
Rols MP, Delteil C, Golzio M et al. In vivo electrically mediated protein and gene transfer in murine melanoma Nat Biotechnol 1998 16: 168–171
Nishi T, Yoshizato K, Yamashiro S et al. High-efficiency in vivo gene transfer using intraarterial plasmid DNA injectionfollowing in vivo electroporation Cancer Res 1996 56: 1050–1055
Goto T, Nishi T, Tamura T et al. Highly efficient electro-gene therapy of solid tumor by using an expression plasmid for the herpes simplex virus thymidine kinase gene Proc Natl Acad Sci USA 2000 97: 354–359
Baba M, Iishi H, Tatsuta M . In vivo electrophoretic transfer of bcl-2 antisense oligonucleotide inhibits the development of hepatocellular carcinoma in rats Int J Cancer 2000 85: 260–266
Bettan M, Ivanov MA, Mir LM et al. Efficient DNA electrotransfer into tumors Bioelectrochemistry 2000 52: 83–90
Lohr F, Lo DY, Zaharoff DA et al. Effective tumor therapy with plasmid-encoded cytokines combined with in vivo electroporation Cancer Res 2001 61: 3281–3284
Yoshizato K, Nishi T, Goto T et al. Gene delivery with optimized electroporation parameters shows potential for treatment of gliomas Int J Oncol 2000 16: 899–905
Susil R, Semrov D, Miklavcic D . Electric field induced transmembrane potential depends on cell density and organization Electro-Magnetobiol 1998 17: 391–399
O'Hare MJ, Ormerod MG, Imrie PR et al. Electropermeabilization and electrosensitivity of different types of mammalian cells In: Neumann E, Sowers AE, Jordan CA, eds Electroporation and Electrofusion in Cell Biology New York: Plenum 1989 319–330
Cemazar M, Jarm T, Miklavcic D et al. Effect of electric-field intensity on electropermeabilization and electrosensitivity of various tumor-cell lines in vitro Electro-Magnetobiol 1998 17: 261–270
Teissie J, Rols MP . Manipulation of cell cytoskeleton affects the lifetime of cell membrane electropermeabilization Ann NY Acad Sci 1994 720: 98–110
Meaking WS, Edgerton J, Harton CW et al. Electroporation-induced damage in mammalian cell DNA Biochim Biophys Acta 1995 1264: 357–362
Coralli C, Cemazar M, Kanthou C et al. Limitations of the reporter green fluorescent protein under simulated tumor conditions Cancer Res 2001 61: 4784–4790
Acknowledgements
This work was supported by the Cancer Research Campaign, Grant SP2292/0102, and the Ministry of Education, Science, and Sport of the Republic of Slovenia. We would like to thank the Advanced Technology Development Group (Gray Cancer Institute) for the production of the custom-made square wave electroporator and imaging system.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cemazar, M., Sersa, G., Wilson, J. et al. Effective gene transfer to solid tumors using different nonviral gene delivery techniques: Electroporation, liposomes, and integrin-targeted vector. Cancer Gene Ther 9, 399–406 (2002). https://doi.org/10.1038/sj.cgt.7700454
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7700454
Keywords
This article is cited by
-
Cytosolic DNA Sensor Upregulation Accompanies DNA Electrotransfer in B16.F10 Melanoma Cells
Molecular Therapy - Nucleic Acids (2016)
-
Tumor radiosensitization by gene therapy against endoglin
Cancer Gene Therapy (2016)
-
The Effect of Millisecond Pulsed Electric Fields (msPEF) on Intracellular Drug Transport with Negatively Charged Large Nanocarriers Made of Solid Lipid Nanoparticles (SLN): In Vitro Study
The Journal of Membrane Biology (2016)
-
Endoglin (CD105) Silencing Mediated by shRNA Under the Control of Endothelin-1 Promoter for Targeted Gene Therapy of Melanoma
Molecular Therapy - Nucleic Acids (2015)
-
Improved Specificity of Gene Electrotransfer to Skin Using pDNA Under the Control of Collagen Tissue-Specific Promoter
The Journal of Membrane Biology (2015)