Abstract
Rhus javanica L. (Anacardiaceae), a dioecious tree with both sexual reproduction and clonal growth, is widely distributed in warm temperate, subtropical, and tropical regions in east Asia. We used allozyme loci and spatial autocorrelation statistics to examine clonal structure and the spatial distribution of allozyme polymorphisms in two Korean populations. Populations of the species maintain moderate levels of allozyme variability (mean He=0.175, GST=0.060), and high levels of multilocus genotypic diversity (mean DG=0.971). Clone-pair distances ranged from 1.4 m to 57.4 m, and had high mean values of 24.0 m and 25.6 m in the two study populations. Approximate genetic patch widths were inferred to be 23–25 m. The results indicated that within populations there is moderate (one study population) or no (other study population) spatial genetic structure among sexually reproduced individuals, and vegetatively reproduced genotypes also are almost randomly distributed. The spatial genetic structure among sexually reproduced trees in the one case is probably caused by limited pollen dispersal in that population, and the lack of structure in the other probably results from the short time elapsed since founding. It appears that clonal reproduction also does not contribute substantially to genetic isolation by distance neither among the sexually reproduced individuals nor the total population. Ramets often establish long distances from their progenitors and thus do not substantially increase the degree of local consanguineous matings.
Similar content being viewed by others
Introduction
Based on a recent review of allozyme literature (Hamrick et al., 1992), there have been relatively few genetic studies of widespread trees (N=11) or trees with both sexual and asexual modes of reproduction (N=15), and with early succession status (N=8). In addition, there are few detailed studies on clonal and spatial genetic structure within populations of woody angiosperms (e.g. Berg & Hamrick, 1994; Shapcott, 1995). In several previous studies, parameters for estimating clonal diversity (e.g. number of clones and clonal diversity within populations) have been calculated without information on population sizes, sampling areas or spatial distributions (e.g. Sherman-Broyles et al., 1992 for three North American sumacs; Yeeh et al., 1996 for Vitex rotundifolia L.f.). Yet all of these factors are important in the dynamics of genetic variation in such species.
Spatial genetic structure within plant populations is primarily determined by the effects of such factors as limited seed and pollen dispersal, isolation in small patches, differential mortality, and microhabitat selection (Levin & Kerster, 1974; Epperson, 1993). For plant species having the capacity to reproduce both sexually and asexually, vegetative reproduction by root suckers or by rhizomes can also have marked effects on the spatial genetic structure of plant populations (e.g. Murawski & Hamrick, 1990; Berg & Hamrick, 1994; Shapcott, 1995). Spatial genetic structure can be analysed in detail using spatial autocorrelation statistics (Sokal & Oden, 1978). Little work, however, has been conducted on the separation of the spatial genetic structure caused by clonal reproduction from that maintained in sexually reproduced individuals (e.g. Chung & Epperson, 1999).
Rhus javanica L. (Anacardiaceae), is a woody species that has both substantial sexual reproduction and clonal spread. It is widely distributed in temperate, subtropical, and tropical regions in east Asia including India. In Korea, the species commonly grows in pine–oak forests in the southern and south-western coastal areas. Like most sumacs, R. javanica is dioecious and insect-pollinated (bees). Fruits (drupes, ≈8–9 mm long) remain attached year-round and may attract frugivorous birds. Here we study in detail the clonal and spatial genetic structure within populations of R. javanica with the aim of determining the spatial distribution of clones and understanding the history of clonal vs. sexual reproduction establishment in the study populations.
Materials and methods
Plant materials
In December 1997, 135 and 150 individuals were mapped, diameters at breast height (d.b.h.) were recorded, and a 10-cm-long branch (one year old) per individual was collected within plots of size 40 m × 60 m (altitude 220 m above sea level, 3% gradient facing SSW) and 40 m × 70 m (altitude 330 m above sea level, 5% gradient facing SSW) at Mount Seonhak, Chinju City, Prov. Gyeongsangnamdo (hereafter referred to as SEO) and at Mount Sansung, Kosung Gun, Prov. Gyeongsangnamdo (SAN), respectively. The two populations are separated by 42 km. In May 1999, a 10 m × 20 m plot within population SAN was excavated and the distance grown by suckers before sending up new trunks (intergrowth) was measured. Both SEO and SAN are located in pine–oak forests in the southern Korean peninsula. Branches were kept on ice, transported to the laboratory, and stored at 4°C until protein extraction.
Enzyme extraction and electrophoresis
Young bark was cut finely, and crushed with a mortar and pestle. A phosphate-polyvinylpyrrolidone extraction buffer (Mitton et al., 1979) was added to the leaf samples to facilitate crushing and to aid enzyme stabilization. The crushed extract was absorbed onto 4 mm × 6 mm wicks cut from Whatman 3MM chromatography paper, which were stored at −70°C until needed for analysis. Electrophoresis was performed using 10% starch gels. Twelve putative loci for R. javanica from six enzyme systems were resolved using a Poulik buffer system, a modification (Haufler, 1985) of Soltis et al. (1983) system 6. These were diaphorase (Dia), fluorescent esterase (Fe-1, Fe-2), peroxidase (Per-1, Per-2), phosphoglucoisomerase (Pgi-1, Pgi-2), phosphoglucomutase (Pgm-1, Pgm-2), and triosephosphate isomerase (Tpi-1, Tpi-2, Tpi-3). Stain recipes followed Soltis et al. (1983), except diaphorase (Cheliak & Pitel, 1984). Putative loci were designated sequentially, with the most anodally migrating isozyme designated 1, the next 2, and so on. Similarly, alleles were designated sequentially with the most anodally migrating alleles designated a.
Data analyses
For the analysis of allozyme diversity, each locus was considered polymorphic if two or more alleles were detected, regardless of their frequencies. Five standard genetic parameters were estimated using the computer program BIOSYS-1 (Swofford & Selander, 1989): percentage polymorphic loci (P), mean number of alleles per polymorphic locus (AP), effective number of alleles per locus (Ae), observed heterozygosity (Ho) and expected heterozygosity or gene diversity (He).
The method of identification of individuals belonging to the same clone uses the total probability (PR) that two identical multilocus genotypes could be produced by sexual reproduction, conditional on the single-locus genotypic frequencies and the assumption of linkage equilibrium (Berg & Hamrick, 1994). Estimates of the probability of getting two identical multilocus genotypes through sexual reproduction were very small in both populations (PR=0.0074 for SEO; 0.0083 for SAN). This indicates that two individuals with identical multilocus genotypes can be assumed to result from clonal reproduction.
The spatial distribution of clones within populations was analysed for the effect of cloning on the formation of spatial patterns. First, the number of clone-pairs sharing the same multilocus genotype was graphed against distance of separation. Secondly, to test the spatial distribution of clones in a way that is not biased by the spatial distribution of all trees themselves, we calculated the spatial autocorrelation statistics for the total number of unlike joins among multilocus genotypes (Sokal & Oden, 1978). A standard normal deviate test statistic (SND) was formed (using the JCSP program of Epperson, 1993), which has an asymptotically standard normal distribution under the null hypothesis of a random spatial distribution. A value of SND <−1.96 would indicate a statistically significant excess of pairs of identical multilocus genotypes (i.e. a deficit of pairs with nonidentical multilocus genotypes) where pairs are separated by a given range of Euclidean distances (Epperson, 1993).
The amount of clonal diversity within and between populations was assessed in three ways. The first measure was the proportion of distinguishable genotypes (Ellstrand & Roose, 1987). This is simply G/N, where G is the number of distinct multilocus genotypes in a population and N is the number of individuals sampled. The second was the probability of obtaining the most common multilocus genotype by chance in each population (PG) (Sherman-Broyles et al., 1992). The third measure of multilocus genotype diversity (DG) was calculated as a modification (Pielou, 1969) of the Simpson index: DG=1 − Σ{[ni(ni − 1)]/[N(N − 1)]}, where ni is the number of individuals of genotype i and N is the total number of individuals in the population. DG is the probability of sampling without replacement two individuals that differ in multilocus genotypes (Gregorius, 1987).
Nei’s (1973, 1977) gene diversity formulae (HT, HS and GST) were used to evaluate the distribution of genetic diversity within populations and differentiation among populations. In addition, a χ2-statistic was used to detect significant differences in allele frequencies among populations for each locus (Workman & Niswander, 1970). It should be noted that Wright’s (1978) FST (GST in Nei’s notation) are most fundamentally measures of genetic fixation. Gregorius & Roberds (1986) demonstrated a desirable measure by introducing a parameter (δ, a weighted average of genetic distances between subpopulations and their components) to measure subpopulation differentiation. This statistic, however, has had relatively little use in the plant allozyme literature (Berg & Hamrick, 1997). Thus, in this study we also interpret GST as measures of genetic differentiation.
Spatial autocorrelation analysis was conducted separately for alleles of each polymorphic locus (Sokal & Oden, 1978). The genotypic data were coded so that allele frequency values of 1.0, 0.5, or 0.0 were assigned to homozygotes for that allele, heterozygotes for that allele, or genotypes with no copies of that allele, respectively. For diallelic loci, only those with allele frequencies less than 0.95 and greater than 0.05 were employed, and then only one allele was considered because the second allele contributes identical information. For multiallelic loci, all alleles at that locus, regardless of their frequencies, were used for the spatial analysis. Each and every pair of individuals was considered as a join and assigned to one of the 10 distance classes according to the Euclidean distance separating the two trees. The boundary ranges of the distance classes were selected in a way that equalized the total number of joins within each. Moran’s I-statistics (Sokal & Oden, 1978) were calculated for each of 10 distance classes by

where N is number of individuals; Wij is an element of the weighting matrix, such that Wij equals to 1.0 if the ith and jth individuals are in the distance class and zero otherwise; Zi=Xi − X, Zj=Xj − X; the variables Xi and Xj are the genotypic scores for the ith and jth individuals, respectively, and X is the mean score for all individuals. Each I-value was used to test for significant deviations from the expected values, E(I )=−1/(N − 1) (Cliff & Ord, 1981), under the null hypothesis of random distribution. A significant positive value of Moran’s I indicates that pairs of individuals in the distance class considered have scores more similar than would be expected if alleles were randomly distributed, whereas a significant negative value indicates that their scores are more dissimilar than expected by chance. Over- all significance of individual correlograms (plots of I-values over the range of distances) was tested using Bonferroni’s criterion (Sakai & Oden, 1983). Statistical analyses were performed using the SAAP program (vers. 4.3) written by D. Wartenberg.
In order to separate out the effects of clonal reproduction, we discarded multiples of the same multilocus genotypes (as these must be clones), after randomly choosing one of each to be retained. This reduced data set, referred to as the set of sexually reproduced genotypes, was also analysed using Moran’s I-statistics as outlined above.
Results
Of the 12 loci examined, five were polymorphic in at least one population. Loci Fe-1, Fe-2, Per-1, Per-2, Pgi-1, Tpi-2 and Tpi-3 were monomorphic in both populations. Estimates of gene diversity within populations (means of P, AP, Ae and He were 41.7%, 1.71, 1.22 and 0.175, respectively) are presented in Table 1. The population SEO has a higher clonal diversity than population SAN: for SEO vs. SAN, G of 68 vs. 56; G/N of 0.504 vs. 0.373; DG of 0.978 vs. 0.963) (Table 1).
Mean d.b.h. in population SEO (2.07 cm ± 1.38 cm [SD]) was significantly larger than that in population SAN (1.39 cm ± 0.98 cm) (unpaired t-test, t=4.75, P < 0.001).
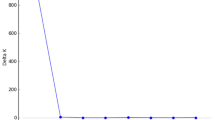
Mean interpair distance of trees with identical multilocus genotypes in population SEO was large (24.01 m ± 13.12 m [SD]) and that in SAN was not significantly different (25.61 m ± 13.75 m) (t=1.375, P > 0.05). The frequency distributions of distances among clones in the two populations are presented in Fig. 1. About half of the multilocus genotypes occurred less than three times within the populations (Table 2). The excavation of the plot in SAN revealed that suckers were highly ramified and the growth distance before sending up new trunks ranged from 1.3 m to 5.3 m (mean 3.45 m ± 1.23 m [SD]). This indicates that clonal growth via suckers followed by disconnections may result in extensive spread into favourable habitats, which may be reflected in the interpair distances of trees with the same multilocus genotypes observed in Fig. 1.
SND statistics for the total number of joins between unlike (nonidentical) multilocus genotypes, with two exceptions, showed no significantly negative values, despite the fact that several sets of distance classes were used, including sets with a distance class less than 4 m. Thus, we can confidently conclude that the distributions of clonal genotypes are essentially random over the area of each of the populations. The two exceptions both occurred in SAN, where the SND was −2.64 for distances <4 m (the first distance class), and −3.00 for distances <6 m. These significant values were caused by an excess of about five pairs of like joins in the first case, and about eight in the second. Thus, there is a slight concentration of clonal reproduction within about 6 m areas in SAN. Still, it is remarkable how widespread clones are in both populations.
According to the criteria described in the Materials and methods, 10 (SEO) and 12 (SAN) alleles were used for single-locus spatial autocorrelation analysis. The spatial autocorrelation coefficients, Moran’s I, for the two populations as well as allele frequencies of these alleles are presented in Tables 3 and 4. For population SEO, Moran’s I-values were significantly different from the expected value (E[I ]=−0.007) in 30 (30%) of 100 cases, and the overall correlogram (a set of I-statistics for all distance classes) was significant for five (50%) of 10 alleles (Table 3). For distance classes 1–4 (0–22 m), 10 significant positive cases were observed, whereas only two (distance class 4) significant negative cases were detected, indicating that some genetic similarity is shared among individuals separated by up to 22 m. A very similar result was observed in population SAN. Moran’s I-values were significantly different from the expected value (E[I ]=−0.007) in 34 (28%) of 120 cases, and the overall correlogram was significant for four (33%) of 12 alleles (Table 4). For distance classes 1–3 (0–20 m), 10 significant positive and one significant negative cases were detected.
Values of I-statistics for population samples excluding multiple clones (the ‘sexually reproduced’ data set) are also shown in Tables 3 and 4. For SEO, the average I-correlogram is almost unchanged by the exclusion of multiple clones. Although the number of significant values is reduced, this could be because the sample size is only about half as large. Nonetheless, the observed result fits the fact that the clonal genotypes themselves are randomly distributed. The situation for SAN is different. The single-locus correlations observed in the total sample disappear when multiple clones are excluded, and the spatial distributions of the sexually reproduced genotypes are essentially random. The autocorrelation observed among clonal genotypes at distances less than 6 m is responsible for all of the autocorrelation observed in the single-locus genotypes in the total sample in the SAN population.
Significant differences in allele frequencies among populations were found for the four (P < 0.001 in each case) polymorphic loci excepting Dia. The GST values ranged from 0.006 for Dia to 0.189 for Pgi-2 with a mean of 0.060, indicating that most of the total variation resided within populations (Table 5).
Discussion
The levels of allozyme variation found in the two populations of R. javanica (He=0.175) are similar to those for populations of two other wide-ranging Rhus species in North America, R. glabra L. (He=0.150) and R. copallina L. (0.150) (Sherman-Broyles et al., 1992). Rhus javanica has more genotypes per population (G=62) than the average (16.1) reported by Ellstrand & Roose (1987) in their review of clonally reproducing species. The average genotypic diversity index for R. javanica is also higher (DG=0.97) than the average (0.61) reported by Ellstrand & Roose. This may be because of large population size and dioecy (outcrossing rate = 1.0). However, the genotypic diversity observed in R. javanica populations is very similar to the averages for the two North American Rhus species (DG=0.95; Sherman-Broyles et al., 1992).
The sets of spatial autocorrelation profiles for single-locus allele frequencies were very similar for the two study populations. There was considerable autocorrelation of tree genotypes in both. In SEO, the clonal genotypes are essentially randomly distributed throughout the area of the population, but the sexually reproduced individuals show genetic isolation by distance at a level comparable to that in a stable population with a Wright’s neighbourhood size of about 125 (Epperson et al., 1999). Seed dispersal distance appears to be large in R. javanica, so pollen flow by bees may be the primary cause of limited gene dispersal. The SEO population has a higher clonal and genetic diversity than SAN. SAN trees have diameters that are much smaller than those in SEO, and in the former few trees have reached reproductive maturity, unlike in SEO. These results may indicate that the SEO population was established earlier than SAN. Theoretical prediction shows that old populations should have more spatial genetic structure than recently established populations (Epperson, 1990). Indeed, SAN might not have been established long enough for isolation by distance to develop, and the initial seedlings must also have had genotypes that were essentially randomly distributed throughout the area of colonization.
The distance at which mean Moran’s I-values first intercept the E(I ) value may represent the average length of the shortest side of an irregularly shaped or sized patch (Sokal, 1979). The mean I-correlograms of the SEO and SAN populations indicate that the approximate patch widths are 23–25 m. Using nine allozymes, Geburek & Tripp-Knowles (1994) estimated a patch width <10 m in a stand (200 m × 200 m area) of Quercus macrocarpa Michx., a sexually reproducing species. Patch widths in both sexually and asexually reproducing tree species, such as Q. margaretta Ashe (≈20 m; Berg & Hamrick, 1994) and Acer saccharum Marsh. (≈20–30 m; Perry & Knowles, 1991), are also similar to the values obtained for R. javanica. These species also appear to have relatively long-distance seed dispersal (acorn dispersal by animal vectors for Q. margaretta and samara dispersal by wind for A. saccharum).
Clonal plants vary considerably in the spatial configurations of genets, from regularly shaped, densely clumped clones with minimal intergrowth (‘phalanx-type’ clones) to irregularly shaped, ramifying clones with more widespread ramets and a tendency for intergrowth and fragmentation or disconnection (‘guerilla-type’ clones) (Lovett Doust, 1981). Examples of ‘phalanx-type’ clones are usually observed in clonally reproducing woody plants (e.g. Xanthoxylum americanum: Reinartz & Popp, 1987; Gleditsia tricanthos: Schnabel & Hamrick, 1990; Quercus laevis and Q. margaretta: Berg & Hamrick, 1994; Quercus havardii: Mayers et al., 1998), whereas ‘guerilla-types’ are found in several herbs (e.g. Aralia nudicaulis: Edwards, 1984; Pteridium aquilinum: Parks & Werth, 1993). The spatial distribution of clones in the study populations indicates that the growth strategy of R. javanica is a ‘guerrilla-type’.
In many plant species, the presence of autocorrelation of clonal genotypes should cause an increase in the incidence of consanguineous matings by proximity. This in turn would increase the spatial autocorrelation among the genotypes of sexually reproduced individuals in the next generation, and hence repeatedly accelerate the development of genetic isolation by distance in future generations. In monoecious species, limited clonal spread may lead to increased effective selfing as well as other types of consanguineous matings, whereas for dioecious species, such as R. javanica, only the latter may occur. Patches of clones may influence attractiveness to pollinators or otherwise increase the frequencies of (consanguineous) matings between adjacent patches of clones that are kin-related because of limited seed dispersal. The presence of clonal patches would also increase the absolute numbers of seed produced through local consanguineous matings by proximity, and this too would increase the degree of genetic isolation by distance among sexually reproduced individuals. The absence of autocorrelation of clonal genotypes precludes these processes in R. javanica.
The mean GST value (0.060) between the two study populations (separated by 42 km) of R. javanica is similar to averages reported for woody species with similar life history characteristics (Hamrick et al., 1992), i.e. wide-ranging species (N=9, 0.033), outcrossing, animal-pollinated species (N=37, 0.099), species with ingested seed dispersal (N=14, 0.051), and species with both sexual and asexual modes of reproduction (N=10, 0.051). A higher GST value for R. javanica would be expected if more strongly isolated populations were included.
In summary, R. javanica maintains moderate levels of allozyme variability, and high levels of multilocus genotypic diversity. The results indicate that clonal reproduction does not contribute substantially to genetic isolation by distance neither among the sexually reproduced individuals nor the total population. Clones are often found long distances from ancestors and thus do not substantially increase the degree of local consanguineous matings.
References
Berg, E. E. and Hamrick, J. L. (1994). Spatial genetic structure of two sandhills oaks: Quercus laevis and Quercus margaretta (Fagaceae). Am J Bot, 81: 7–14.
Berg, E. E. and Hamrick, J. L. (1997). Quantification of genetic diversity at allozyme loci. Can J For Res, 27: 415–424.
Cheliak, W. M. and Pitel, J. P. (1984). Techniques for starch gel electrophoresis of enzymes from forest tree species. Information Report PI-X-42, Petawawa National Forestry Institute, Agriculture Canada, Canadian Forestry Service, pp. 1–49. Chalk River, Ontario.
Chung, M. G. and Epperson, B. K. (1999). Spatial genetic structure of clonal and asexual reproduction in populations of Adenophora grandiflora (Campanulaceae). Evolution, 53: 1068–1078.
Cliff, A. D. and Ord, J. K. (1981). Spatial Processes — Methods and Applications, Pion, London.
Edwards, J. (1984). Spatial pattern and clone structure of the perennial herb, Aralia nudicaulis L. (Araliaceae). Bull Torrey Bot Club, 111: 28–33.
Ellstrand, N. C. and Roose, M. L. (1987). Patterns of genotypic diversity in clonal plant species. Am J Bot, 74: 123–131.
Epperson, B. K. (1990). Spatial autocorrelation of genotypes under directional selection. Genetics, 124: 757–771.
Epperson, B. K. (1993). Recent advances in correlation analysis of spatial patterns of genetic variation. Evol Biol, 27: 95–155.
Epperson, B. K., Huang, Z. and Li, T. (1999). Measures of spatial structure in samples of genotypes for multiallelic loci. Genet Res, 73: 251–261.
Geburek, T. and Tripp-Knowles, P. (1994). Genetic architecture in bur oak, Quercus macrocarpa (Fagaceae), inferred by means of spatial autocorrelation analysis. Pl Syst Evol, 189: 63–74.
Gregorius, H. -R. (1987). The relationship between the concepts of genetic diversity and differentiation. Theor Appl Genet, 74: 397–401.
Gregorius, H. -R. and Roberds, J. H. (1986). Measurement of genetical differentiation among subpopulations. Theor Appl Genet, 71: 826–834.
Hamrick, J. L., Godt, M. J. W. and Sherman-Broyles, S. L. (1992). Factors influencing levels of genetic diversity in woody plant species. New Forests, 5: 95–124.
Haufler, C. H. (1985). Enzyme variability and modes of evolution in Bommeria (Pteridaceae). Syst Bot, 10: 92–104.
Levin, D. A. and Kerster, H. W. (1974). Gene flow in seed plants. Evol Biol, 7: 139–220.
Lovett Doust, L. (1981). Population dynamics and local specialization in a clonal perennial (Ranunculus repens). I. The dynamics of ramets in contrasting habitats. J Ecol, 69: 743–755.
Mayers, S. G., Mcginley, M. A. and Werth, C. R. (1998). Clonal population structure and genetic variation in sand-shinnery oak, Quercus havardii (Fagaceae). Am J Bot, 85: 1609–1617.
Mitton, J. B., Linhart, Y. B., Sturgeon, K. B. and Hamrick, J. L. (1979). Allozyme polymorphisms detected in mature needle tissue of ponderosa pine. J Hered, 70: 86–89.
Murawski, D. A. and Hamrick, J. L. (1990). Local genetic and clonal structure in the tropical terrestrial bromeliad, Aechmea magdalenae. Am J Bot, 77: 1201–1208.
Nei, M. (1973). Analysis of gene diversity in subdivided populations. Proc Natl Acad Sci USA, 70: 3321–3323.
Nei, M. (1977). F-statistics and analysis of gene diversity in subdivided populations. Ann Hum Genet, 41: 225–233.
Parks, J. C. and Werth, C. R. (1993). A study of spatial features of clones in a population of bracken fern, Pteridium aquilinum (Dennstaedtiaceae). Am J Bot, 80: 537–544.
Perry, D. J. and Knowles, P. (1991). Spatial genetic structure within three sugar maple (Acer saccharum Marsh.) stands. Heredity, 66: 137–142.
Pielou, C. E. (1969). An Introduction to Mathematical Ecology. Wiley-Interscience, New York.
Reinartz, J. and Popp, J. (1987). Structure of clones of Northern prickly ash (Xanthoxylum americanum). Am J Bot, 74: 415–428.
Sakai, A. K. and Oden, N. L. (1983). Spatial pattern of sex expression in silver maple (Acer saccharum L.): Morista’s index and spatial autocorrelation. Am Nat, 122: 489–508.
Schnabel, A. and Hamrick, J. L. (1990). Organization of genetic diversity within and among populations of Gleditsia triacanthos (Leguminosae). Am J Bot, 77: 1060–1069.
Shapcott, A. (1995). The spatial genetic structure in natural populations of the Australian temperate rain forest tree Atherosperma moschatum (Labill.) (Monimiaceae). Heredity, 74: 28–38.
Sherman-Broyles, S. L., Gibson, J. P., Hamrick, J. L., Bucher, M. A. and Gibson, M. J. (1992). Comparisons of allozyme diversity among rare and widespread Rhus species. Syst Bot, 17: 551–559.
Sokal, R. R. (1979). Ecological parameters inferred from spatial correlograms. In: Petil, G. P. and Rosenzweig, M. L. (eds) Contemporary Quantitative Ecology and Related Ecometrics, pp. 167–196. International Cooperative Publishing House, Fairland, NY.
Sokal, R. R. and Oden, N. L. (1978). Spatial autocorrelation in biology. 1. Methodology. Biol J Linn Soc, 10: 199–249.
Soltis, D. E., Haufler, C. H., Darrow, D. C. and Gastony, G. J. (1983). Starch gel electrophoresis of ferns: a compilation of grinding buffers, gel and electrode buffers, and staining schedules. Am Fern J, 73: 9–27.
Swofford, D. L. and Selander, R. B. (1989). Biosys-1. A computer program for the analysis of allelic variation in population genetics and biochemical systematics. Release 1.7. University of Illinois, Urbana, IL.
Workman, P. L. and Niswander, J. D. (1970). Population studies on southern Indian tribes. II. Local genetic differentiation in the Papago. Am J Hum Genet, 22: 24–49.
Wright, S. (1978). Evolution and the Genetics of Populations, vol. 4, Variability Within and Among Natural Populations. University of Chicago Press, Chicago, IL.
Yeeh, Y., Kang, S. S., Chung, H. G., Chung, M. S. and Chung, M. G. (1996). Genetic and clonal diversity in Korean populations of Vitex rotundifolia (Verbenaceae). J Plant Res, 109: 161–168.
Acknowledgements
This research was supported in part by a Korea Science Engineering Foundation grant (KOSEF 96-0500-006-2) to M.G.C. and a McIntire-Stennis (project no. 1774) grant to B.K.E.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chung, M., Chung, J., Chung, M. et al. Spatial distribution of allozyme polymorphisms following clonal and sexual reproduction in populations of Rhus javanica (Anacardiaceae). Heredity 84, 178–185 (2000). https://doi.org/10.1046/j.1365-2540.2000.00660.x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1046/j.1365-2540.2000.00660.x
Keywords
This article is cited by
-
Maintenance of Phenotypic and Genotypic Diversity in Managed Populations of Stenocereus Stellatus (Cactaceae) by Indigenous Peoples in Central Mexico
Biodiversity and Conservation (2006)
-
Estimating dispersal from short distance spatial autocorrelation
Heredity (2005)
-
Testing for clonal propagation
Heredity (2005)