Abstract
Matrix metalloproteinases constitute one of the major extracellular matrix degrading enzymic families implicated in cancer development. Stromelysin-3 in particular, a member of the matrix metalloproteinases belonging to the stromelysins’ subgroup, seems to be closely related to invasiveness and tumor progression. In this study, we proceeded to the evaluation of stromelysin-3 protein’s expression in paraffin sections of 133 cases of invasive breast carcinomas and statistically estimated its relations with known clinicopathological prognostic parameters and patients’ survival, proliferation markers Ki-67 and TopoIIα and the antiapoptotic protein bcl-2. Presence of stromelysin-3 was immunodetected, in the 73% of our cases, in stromal cells (65%) and in epithelial tumor cells (26.26%). Stromelysin-3 epithelial positivity presented statistically significant correlations with TopoIIα and Ki-67 proliferation indices (P = .042 and P = .031, respectively) and worse disease outcome through multivariate statistics (P = .014). Stromelysin-3 fibroblastic expression was significantly associated with nuclear grade (P = .024), ductal histological type (P = .037), TopoIIα (P = .001) and Ki-67 (P = .019), inversely with bcl-2 protein (P = .027) and with adverse overall survival through univariate analysis (P = .017). The subgroup of patients with stromelysin-3 co-expression in stromal and malignant epithelial cells showed statistically significant associations with Ki-67 and TopoIIα (P = .019, P < .0001, respectively), an inverse one with bcl-2 protein (P = .027) and furthermore with impaired survival (P = .002) through multivariate analysis. In conclusion, stromelysin-3 protein expression correlated with proliferation indices TopoIIα and Ki-67 and the anti-apoptotic protein bcl-2, data confirming stromelysin-3’s contribution to breast cancer progression. Moreover its expression was shown to have a direct negative effect on patients’ survival, especially in the subgroup of patients with simultaneous epithelial and stromal expression.
Similar content being viewed by others
INTRODUCTION
Over the past decades, the international scientific community has shown an increasing interest in the potential role of extracellular matrix (ECM), as well as in the enzymes responsible for its remodeling, in the process of tumor invasion and metastasis, because its active destruction is a sine qua non for malignancy to develop (1). One of the major extracellular matrix-degrading enzymic families implicated in cancer development is matrix metalloproteinases (MMPs), also called matrixins (2), a group of zinc and calcium-dependent endopeptidases, with a broad spectrum of proteolytic activity toward extracellular matrix components (3, 4, 5). The 21 distinct MMPs so far identified, can be grouped, on the basis of their structure and substrate specificity, into the following subclasses: (a) collagenases (MMP-1, −8, −13), (b) gelatinases (MMP-2, −9), (c) stromelysins (MMP-7 or matrilysin, −3 or ST-1, −10 or ST-2, −11 or stromelysin-3), (d) membrane-type MMPs (MT1-, MT2-, MT3-, MT4-, MT5-MMP) and other MMPs (6). Disposing overlapping yet distinct functions, as a whole, they can degrade practically every extracellular matrix component, acting always under a multistep regulatory system including specific tissue inhibitors of metalloproteinases and inducers (e.g., extracellular matrix metalloproteinase inducer) (2).
Stromelysin-3 (MMP-11) in particular, has been originally described and isolated from fibroblastic cells surrounding invasive breast carcinoma (7). Ever since, it remains an intriguing target for further study due to its persistently found overexpression in almost every aggressive malignancy, although its putative mature forms seem to exhibit little or no obvious proteolytic activity towards major extracellular matrix components (8). Its predicted structure shares common features with that of other stromelysins and collagenases. However, unlike all other MMPs, which are secreted in proenzyme form, stromelysin-3 along with membrane type 1-MMP, are activated before secretion by Golgi-associated furin-like proteases (9, 10, 11). Furthermore, another originality of stromelysin-3 is observed on the functional level, where its active form is unable to hydrolyze extracellular matrix molecules and its action is restricted to the cleavage of β-casein (8), α2-macroglobulin (8), and serine proteinase inhibitors such as α1-proteinase inhibitor and α1-antitrypsin (12, 13, 14).
Stromelysin-3′s gene, located on chromosome 22g11.2 (10, 15), is being expressed at high levels, predominantly by peritumoral fibroblasts, in almost all invasive breast carcinomas and in a number of breast cancer metastases. Moreover, stromelysin-3 expression is probably implicated in early breast cancer progression, an observation emerging from its presence in in situ tumors of the comedo type, of which the majority become invasive (16). Further studies tend to link high stromelysin-3 expression to poor prognosis in breast cancer (17).
The aim of this study was to investigate the expression of stromelysin-3 in a series of invasive breast carcinomas and assess any statistical correlation with known clinicopathological prognostic parameters, the expression of TopoIIα, Ki-67, bcl-2 and patients’ survival.
MATERIALS AND METHODS
Patients and Tissue Collection
The present specimen comprise 133 cases of primary invasive breast carcinomas from patients undergoing surgery between 1992–1993, obtained from the archives of the Department of Pathology of Athens University Medical School. In all 133 cases, mastectomy or lobectomy, with axillary lymph node evacuation, had been the primary treatment. After surgical operation, patients with positive lymph nodes and premenopausal patients with tumor size more than 1 cm and negative lymph nodes received classical chemotherapy (CMF). All patients, regardless of hormonal status, were treated with tamoxifen. The age of the patients’ group ranged from 25 to 87 years, (mean age: 57.4 ± 13.2, median: 57 years) and they have been followed-up at 6-month intervals, for a median period of 77 months, presenting a mean survival period of 70.9 months (7–94 months). Survival data were available for 128 cases. Determination of tissues’ pathological features was performed as previously described (18). Fresh tissue samples were immediately fixed in 10% neutral formalin buffered followed by paraffin embedment and storage at room temperature.
Immunohistochemistry
Immunohistochemical staining for stromelysin-3 was performed on 4 μm thick formalin-fixed paraffin sections, using an avidin-biotin immunoperoxidase technique, after overnight heating at 60°C and subsequent deparaffinization in xylene and rehydration through graded alcohols. After quenching of endogenous peroxidase activity using a methanol/hydrogen peroxide solution (0.3% in TBS for 30 min), we proceeded to autoclave heat-mediated antigen retrieval in 10 mm, pH = 6 citrate buffer and blockage of nonspecific binding by incubation in 10% normal horse serum in TBS (Vector Lab, Burlingame, CA, USA) for 30 min at room temperature. Subsequently, sections were incubated overnight, at 4°C in a humidified chamber, with the monoclonal primary antibody 5ST-4A9, raised against the hemopexin-like domain of human stromelysin-3 (kindly offered to us by Prof. P. Chambon, IGBMC, Strasburg, France) at a dilution of 1:1000 in TBS. Sections were then incubated in biotinylated horse anti-mouse secondary antibody diluted 1:150 in bovine serum albumin, for 30 min at room temperature followed by peroxidase-conjugated avidin-biotin complexes (Vectastain Elite ABC kit, Vector Lab, Burlingame, CA, USA) in a 30 min incubation at room temperature and addition of 3,3′-diaminobenzidine tetrachlorohydrate (3,3′-diaminobenzidine), to achieve visualization of the requested antigen. Nuclei were counterstained using Harris’ hematoxylin.
The other, herein assessed, immunomarkers, meaning ER, PR, Ki-67, TopoIIα, and bcl-2 proteins, they all have been detected as previously described (18). Slides treated in the same manner, yet omitting the step of primary antibody application served as negative controls
Evaluation of Immunostaining Scores
Immunostaining evaluation of stromelysin-3 was performed by two independent pathologists through light microscopic observation, using a semiquantitative method. The score resulted as the average of 10 distinct high-power fields observed under x400 magnification. From this perspective, we observed and evaluated two distinct expression patterns, with characteristic granular appearance: (a) cancer cell cytoplasmic, scored as 0 (0%), 1 (≤10%) and 2 (more than 10%). These cut points were employed due to the small number of positive cancer cells, and (b) peritumoral fibroblastic, scored as 0 (negative, 0%), 1 (weak, ≤10%), 2 (moderate, 11–30%) and 3 (strong, more than 30%). Except for the case of TopoIIα, of which low expression levels forced us to use image analysis assessment on a total number of 500 neoplastic nuclei, all the other indicators were microscopically observed and semiquantitavely scored, as previously described (18).
Statistical Analysis
Correlations between stromelysin-3 expression levels and the other assessed variables were investigated using Pearson’s chi-square test or Fisher’s Exact test (as appropriate), except for the case of TopoIIα, of which the distribution indicated the use of Kruskall-Wallis test (non-parametric statistical method). The effect of stromelysin-3 differential expression on postoperative survival rates was assessed using both univariate (log-rank test) and multivariate (stepwise forward Cox’s proportional hazard regression model with 95% hazard ratio’s confidence interval) analysis, with the latter model adjusted for all the assessed parameters (lymph node status, age, stage and size of tumors, nuclear grade, histologic type, ER and PR status, Ki-67, TopoIIα and bcl-2 protein expression levels).
RESULTS
Immunohistochemical Expression of Stromelysin-3
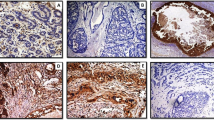
Cases exhibiting various positivity for stromelysin-3 protein in the peritumoral fibroblast-like cells or the neoplastic epithelium, were altogether 97 out of 133 (73%). These two distinct patterns, were, in some cases, co-existing (n = 25) and in others the expression was either stromal or epithelial only (n = 61 and n = 10, respectively). Stromelysin-3 stromal expression was detected in 86 of 133 cases (65%) presenting various positivity limited to reactive stromal cells within the tumor (Fig. 1A). This certain pattern associated significantly with tumor histological type, detected more often in ductal carcinomas than in lobular ones (P = .037, Table 1). A significant correlation was also revealed between intense stromelysin-3 protein expression levels and increasing nuclear grade (P = .024, Table 1). Furthermore, stromelysin-3′s expression exhibited significant relations, which, to the best of our knowledge, are being mentioned for the first time, with TopoIIα (P = .001, Fig. 2A), Ki-67 (P = .019, Table 1, Fig. 2B) and an inverse one with bcl-2 antiapoptotic protein (P = .027, Table 1, Fig. 2C). Univariate analysis demonstrated a worse survival of patients with stromelysin-3 expression in stromal cells (P = .017).
To the best of our knowledge, this study is the first report of stromelysin-3 expression in cancer cells, with statistically significant findings, found persistently expressed in 35 out of the 133 cases (26%) (Fig. 1B). Stromelysin-3 cytoplasmic expression in cancer cells showed a parallel statistical significant correlation with TopoIIα (P = .042, Fig. 2A) and Ki-67 (P = .031, Table 1, Fig. 2B). Furthermore, stromelysin-3′s expression in the malignant epithelial cells, was correlated with impaired survival, confirmed through both the univariate (P = .023) and multivariate analysis (P = .014) (Fig. 3, Table 2). In particular, multivariate analysis demonstrated that patients expressing high stromelysin-3 levels had even worse survival (P = .005). The Cox model found for survival, also revealed standard prognostic parameters such as LN (P = .008), age (P = .025) and PR (P = .0007).
The subgroup of patients with co-expression of stromelysin-3 in stromal and cancer cells exhibited a parallel relation of unequivocal statistical significance with TopoIIα (P < .0001), the same with Ki-67 (P = .019), inversewith the bcl-2 (P = .027) (data not shown) and a significant correlation with worst patients’ outcome through both univariate (P = .028) and multivariate (P = .002, Table 3) statistics.
DISCUSSION
Co-expression of several extracellular matrix degradative enzymes secures the presence of a favorable environment for tumor growth and its subsequent dissemination and metastasis. Among these enzymes the various MMPs are believed to play a pivotal role in promoting tumor progression. In our present study, we proceeded to an immunohistochemical assessment of stromelysin-3 protein expression in a series of 133 invasive breast carcinomas of conventional morphology. Stromelysin-3 was predominantly detected in stromal cells within the tumor (65%) and in a lesser, yet persistent degree in cancer cells (26%). It is well known that the vast majority of literature regarding stromelysin-3 supports the predominant if not exclusive fibroblastic production and localization of this particular MMP (19, 20, 21). However, there are preliminary reports that mention stromelysin-3 cytoplasmic localization in cancer cells in a series of metaplastic mammary carcinomas (22) and detection of stromelysin-3 mRNA in the tumor epithelium of breast cancer tissues (19). A possible explanation accounting for the above conflicting data may rest on the fact that stromelysin-3 is specifically expressed by fibroblastic cells located close to malignant cells, thus suggesting the existence of some kind of cross-talk between the two cell types and a paracrine function for stromelysin-3 (23). Furthermore, although the various members of the MMP family, are mainly expressed by reactive stromal cells, there is a growing body of evidence, supporting epithelial production of several MMPs (24, 25).
Both the epithelial and stromal expression patterns of stromelysin-3 protein, observed in our study, showed statistically significant associations with impaired patients’ survival. More precisely the negative effect of high stromelysin-3 epithelial expression on patients’ outcome was demonstrated by both univariate (P = .023) and multivariate analysis (P = .014). The previous studies reporting epithelial stromelysin-3 localization do not mention such associations with disease outcome (19, 22). Nevertheless, reports on other MMPs supporting our findings, associate their detection in cancer cells with worse prognosis (25). On the other hand, stromal stromelysin-3 expression was found to have a prognostic value regarding poor disease outcome in univariate analysis (P = .017), finding similar to those of previous reports (20, 22). The subgroup of patients with simultaneous stromelysin-3 epithelial and stromal localization exhibited the worst disease prognosis (P = .002).
An essential observation of this study was the obvious correlations of stromelysin-3 detection in either cancer or stromal cells with the well established indices of cellular proliferation TopoIIα (P = .042, P = .001, respectively) and Ki-67 (P = .031, P = .019, respectively). These certain findings, to the best of our knowledge, are being reported for the first time. TopoIIα, in particular, serves as an index of major credibility regarding active cell proliferation, due to the fact that it is mainly expressed during S1 and G2 phases of the cell cycle, implicated in the opening of DNA strands and known to be parallelly associated with nuclear grade (18). The above mentioned data support the notion of a potential role of stromelysin-3 in cell proliferation, which is further reinforced by the statistically significant correlation of high stromelysin-3 levels with increasing nuclear grade (P = .024) observed in our investigation. Furthermore, Tetû et al. have previously reported a significant association of stromelysin-3 high levels with tumor cell aneuploidy (20). Although there is experimental evidence demonstrating that stromelysin-3 did not directly influence cell proliferation rates (26, 27), the same authors finally conclude that stromelysin-3 probably releases or activates growth factors or cytokines stored in the extracellular matrix (27). In fact they have observed that stromelysin-3 exhibited its tumor promoting effect synergistically with matrigel containing growth factors and not when the latter is depleted of them (27, 28).
Bcl-2 oncoprotein is assumed to be involved in the control of the apoptotic pathway; it is believed to be important in suppressing apoptosis (29). On the other hand, in vitro and in vivo observations indicate that stromelysin-3 expression promotes tumor take in nude mice, presumably by favoring cancer cell survival in a tissue environment initially not permissive for tumor growth (26). Besides, Boulay et al. (30) reported that syngeneic tumors developed in stromelysin-3 deficient mice present an enhanced apoptotic rate compared with wild type ones, indicating a role for stromelysin-3 in the maintenance and survival of cancer cells through inhibition of extracellular matrix-induced apoptosis. These observations are supported by our findings associating stromelysin-3 protein expression with poor patients’ outcome.
Despite the above concluded similar effect of stromelysin-3 and bcl-2 proteins on cell survival, we have demonstrated an inverse association between fibroblastic stromelysin-3 expression and bcl-2 levels (P = .027). This observation, although paradoxical at first, derives support from previous studies that link the antiapoptotic bcl-2 protein to features indicating favorable prognosis in mammary carcinomas (29, 31). It cannot be discerned from correlative studies whether bcl-2 is involved directly in contributing to an indolent phenotype or is simply an epiphenomenon (29).
Another correlation observed in our series was the one between stromelysin-3 stromal expression and the invasive ductal carcinoma. This relation has been mentioned by other observers too and it probably reflects the more active fibroblastic activity observed in ductal carcinomas, although the possible secretion of unknown paracrine factors specific for this certain histologic subtype cannot be ruled out (20).
CONCLUSION
In conclusion, stromelysin-3 protein expression correlated with proliferation indices TopoIIα and Ki-67 and the anti-apoptotic protein bcl-2, data confirming stromelysin-3′s contribution to breast cancer progression. Moreover its expression was shown to have a direct negative effect on patients’ survival, especially in the subgroup of patients with simultaneous epithelial and stromal expression.
References
Liotta LA, Steeg PS, Stetler-Stevenson WG . Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell 1991; 64: 327–336.
Nagase H, Woessner JF Jr . Matrix metalloproteinases, minireview. J Biol Chem 1999; 274: 21491–4.
Matrisian LM . The matrix-degrading metalloproteinases. Bioassays 1992; 14: 455–463.
Docherty AJP, O’ Connell J, Crabbe T, Angal S, Murphy G . The MMPs and their natural inhibitors: prospects for treating degenerative tissue disorders. Trends Biotechnol 1992; 10: 200–207.
Birkedal-Hansen H . Proteolytic remodeling of extracellular matrix. Curr Opin Cell Biol 1995; 7: 728–735.
Mignatti P, Rifkin DB . Nonenzymatic interactions between proteinases and the cell surface: novel roles in normal and malignant cell physiology, review. Adv Cancer Res 1000; 78: 103–157.
Basset P, Belocq JP, Wolf C, Stoll I, Hutin P, Limacher JM, et al. A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature 1990; 348: 699–704.
Pei D, Majmudar G, Weiss SJ . Hydrolytic inactivation of a breast carcinoma cell-derived serpin by human ST-3. J Biol Chemistry 1994; 269: 25849–25855.
Westermarck J, Kähäri VM . Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J 1999; 13: 781–792.
Forget M-A, Desrosiers R, Béliveau R . Physiological roles of matrix metalloproteinases: implications for tumor growth and metastasis, review. Can J Physiol Pharmacol 1999; 77: 465–480.
Pei D, Weiss SJ . Furin-dependent intracellular activation of the human ST-3 zymogen. Nature 1995; 375: 244–247.
Kähäri VM, Saariahlo-Kere U . Matrix metalloproteinases in skin. Exp Dermatol 1997; 6: 199–213.
Shapiro SD . Matrix metalloproteinase degradation of extracellular matrix: biological consequences. Curr Opin Cell Biol 1998; 10: 602–608.
Woessner JF . The matrix metalloproteinase family. In: Parks WC, Mecham RP, editors. Matrix metalloproteinases. San Diego: Academic Press; 1998. pp.1–4.
Levy A, Zucman J, Delattre O, Mattei MG, Rio MC, Basset P . Assignment of the human stromelysin-3 (ST-3) gene to the q11.2 region of chromosome 22. Genomics 1992; 13: 881–883.
Wolf C, Rouyer N, Lutz Y, Adida C, Loriot M, Bellocq J-P, et al. Stromelysin-3 belongs to a subgroup of proteinases expressed in breast carcinoma fibroblastic cells and possibly implicated in tumor progression. Proc Natl Acad Sci USA 1993; 90: 1843–1847.
Engel G, Heselmeyer K, Auer G, Backdahl M, Eriksson E, Linder S . Correlation between stromelysin-3 mRNA level and outcome of human breast cancer. Int J Cancer 1994; 58: 830–835.
Nakopoulou L, Lazaris A, Kavantzas N, Alexandrou P, Athanassiadou P, Keramopoulos A, et al. DNA Topoisomerase IIα immunoreactivity as a marker of tumor aggressiveness in invasive breast cancer. Pathobiology 2000; 68: 137–143.
Escot C, Zhao Yang, Puech C, Rochefort H . Cellular localisation by in situ hybridisation of cathepsin D, stromelysin 3 and urokinase plasminogen activator RNAs in breast cancer. Breast Cancer Res Treat 1996; 38: 217–226.
Tetû B, Brisson J, Lapointe H, Bernard P . Prognostic significance of stromelysin 3, gelatinase A and urokinase expression in breast cancer. Hum Pathol 1998; 29: 979–985.
Chenard M-P, O’Siorain L, Shering S, Rouyer N, Lutz Y, Wolf C, et al. High levels of stromelysin-3 correlate with poor prognosis in patients with breast carcinoma. Int J Cancer 1996; 69: 448–451.
Ahmad A, Hanby A, Dublin E, Poulsom R, Smith P, Barnes D, et al. Stromelysin 3: an independent prognostic factor for relapse-free survival in node-positive breast cancer and demonstration of novel breast carcinoma cell expression. Am J Pathol 1998; 152: 721–728.
Basset P, Okada A, Chenard MP, Kannan R, Stoll I, Anglard P, et al. Matrix metalloproteinases as stromal effectors of human carcinoma progression: therapeutical implications. Matrix Biol 1997; 15: 534–541.
Lebeau A, Nerlich AG, Saver U, Lichtinghagen R, Zohrs U . Tissue distribution of major matrix metalloproteinases and their transcripts in human breast carcinomas. Anticancer Res 1999; 19: 4257–4264.
Talvensaari-Mattila A, Pääkkö P, Höyhtya M, Blanco-Sequeiros G, Turpeenniemi-Hujanen T . Matrix metalloproteinase-2 immunoreactive protein. Cancer 1998; 83: 1153–1162.
Nöel AC, Lefebvre O, Maquoi E, VanHoorde L, Chenard M-P, Mareel M, et al. Stromelysin-3 expression promotes tumor take in nude mice. J Clin Invest 1996; 97: 1924–1930.
Nöel AC, Boulay A, Kebers F, Kannan R, Hajitou A, Calberg-Bacq CM, et al. Demonstration in vivo that stromelysin-3 functions through its proteolytic activity. Oncogene 2000; 19: 1605–1612.
Masson R, Lefebvre O, Nöel A, El Fahime M, Chenard M-P, Wendling C, et al. In vivo evidence that the stromelysin-3 metalloproteinase contributes in paracrine manner to epithelial cell malignancy. J Cell Biol 1998; 140: 1535–1541.
Nakopoulou L, Michalopoulou A, Giannopoulou I, Tzonou A, Keramopoulos A, Lazaris AC, et al. Bcl-2 protein expression associated with a prognostically favorable phenotype in breast cancer irrespective of p53 immunostaining. Histopathology 1999; 34: 310–319.
Boulay A, Masson R, Chenard MP, El Fahime M, Cassard L, Bellocq JP, et al. High cancer cell death in syngeneic tumors developed in host mice deficient for the stromelysin-3 matrix metalloproteinase. Cancer Res 2001; 61: 2189–2193.
Zapata JM, Krajewska M, Krajewski S, Huang R-P, Takayama S, Wang H-G, et al. Expression of multiple apoptosis-regulatory genes in human breast cancer cell lines and primary tumors. Breast Cancer Res Treat 1998; 47: 129–140.
Acknowledgements
The present study was granted by funds of the Greek Ministry of Development. The authors would like to thank: Prof. P. Chambon and Dr M-C Rio for providing us with the monoclonal antibody 5ST-4A9 against human stromelysin-3.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakopoulou, L., Panayotopoulou, E., Giannopoulou, I. et al. Stromelysin-3 Protein Expression in Invasive Breast Cancer: Relation to Proliferation, Cell Survival and Patients’ Outcome. Mod Pathol 15, 1154–1161 (2002). https://doi.org/10.1097/01.MP.0000037317.84782.CD
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1097/01.MP.0000037317.84782.CD
Keywords
This article is cited by
-
Inflammatory and microenvironmental factors involved in breast cancer progression
Archives of Pharmacal Research (2013)
-
Effect of the Expression of Matrix Metalloproteases and Their Tissue Inhibitors on Survival of Patients with Resectable Colorectal Cancer
Digestive Diseases and Sciences (2012)
-
Matrix metalloproteinase-11 overexpressed in lobular carcinoma cells of the breast promotes anoikis resistance
Virchows Archiv (2011)
-
Gli1 enhances migration and invasion via up-regulation of MMP-11 and promotes metastasis in ERα negative breast cancer cell lines
Clinical & Experimental Metastasis (2011)
-
Metastatic canine mammary carcinomas can be identified by a gene expression profile that partly overlaps with human breast cancer profiles
BMC Cancer (2010)