Abstract
Recent evidence suggests that a loss of expression of caveolin in the stromal compartment (sCav-1) of human invasive breast carcinoma (IBC) may be a predictor of disease recurrence, metastasis and poor outcome. At present, there is little knowledge regarding the expression of sCav-1 at the metastatic sites. We therefore studied sCav-1 expression in IBCs and in their axillary lymph nodes to seek a correlation with cancer metastasis. 189 consecutive invasive IBCs (53 with axillary lymph node metastases and 136 without) were studied by immunohistochemistry, using a rabbit polyclonal anti-Cav-1 antibody. In IBCs sCav-1 was evaluated in fibroblasts scattered in the tumor stroma whereas in lymph nodes sCav-1 was assessed in fibroblast-like stromal cells. For the first time, we observed a statistically significant progressive loss of sCav-1 from normal/reactive axillary lymph nodes of tumors limited to the breast to metastatic axillary lymph nodes, through normal/reactive axillary lymph nodes of tumors with axillary metastatic spread. These data indicate that Cav-1 expressed by the stromal compartment of lymph nodes, somehow, may possibly contribute to metastatic spread in IBC.
Similar content being viewed by others
Introduction
Breast cancer is a heterogeneous disease, the most frequent malignant tumor in women worldwide, with more than 2 million new cases and nearly 630.000 estimated deaths in 20181. Studies have traditionally focused on elucidation of the events arising in the cancerous epithelial cells with little attention to the role of the surrounding stroma or microenvironment2. At present, fibroblasts, blood vessels and extracellular components are thought to be key regulators of cancer progression. Indeed, modified fibroblasts, the so-called “cancer associated fibroblasts” (CAFs), modulate extracellular matrix degradation3 and regulate proliferation of the epithelial cancer cells within the primary tumor site, via the production of numerous growth factors and cytokines, including HGF, EGF, IGFs, IGFBPs, b-FGF and TGF-β4. The tumor microenvironment likely plays a role also at metastatic sites, to re-establish the conditions that disseminated tumor cells (DTCs) require for growth5. The term “metastatic niche” has been coined to refer to this phenomenon and has been described for visceral secondary sites but also for lymph nodes: here, fibroblast-like stromal cells, together with bone marrow derived cells, play a crucial role to form these permissive compartments6. At present, the precise complex mechanisms through which the stroma orchestrates tumor progression are not fully elucidated. In recent decades, increasing attention has been paid to the expression of caveolin-1 (Cav-1) in the tumor microenvironment. Cav-1 is the essential constituent protein of caveolae, specialized flask-shaped invaginations of the plasma membrane, that act as signal transduction hubs by providing compartmentalization of intracellular signaling molecules7. Cav-1 is expressed on the cell membrane of endothelial cells, adipocytes, smooth muscle cells and type 1 pneumocytes. Inside caveolae, Cav-1 binds to G-protein subunits, tyrosine kinases and endothelial nitric oxide synthase, thus regulating several signaling pathways8. Mouse models studies demonstrated that caveolins play an important role in a number of human diseases such as atherosclerosis, muscular dystrophy, diabetes and cancer9. The role for this protein in the process of cell transformation was reported in 1989, when Cav-1 was identified as a substrate for v-Src in transformed chicken embryo fibroblasts10. Later research suggested Cav-1 gene as tumor suppressor as well as proto-oncogene: downregulation is associated to colon and ovarian carcinomas or sarcomas instead upregulation to prostate, lung, esophageal, bladder and papillary thyroid carcinomas11.
In breast cancer, Cav-1 gene has been suggested as tumor suppressor12; however, aggressive breast cancer subtypes overexpress Cav-1 protein, supporting an oncogenic role13. Thus, the role of Cav-1 in tumorigenesis remains contentious. Interestingly, a number of recent studies have investigated the role of Cav-1 in the tumor microenvironment, describing stromal Cav-1 (sCav-1) as a marker of the CAF phenotype in human breast cancer14. In particular, the decreased expression of sCav-1 is associated with poor prognosis15,16,17. Loss of sCav-1 also predicts poor outcome18, in particular in the subgroup of basal-like breast cancers19. In addition, a striking correlation between loss of sCav-1 and early progression from in situ to invasive breast carcinoma has recently been reported20. However, most of the studies have concentrated so far on sCav-1 in primary tumor and there is little knowledge about the expression of sCav-1 at the metastatic sites, in breast cancer or in other tumor models. The aim of this study was to investigate the expression of Cav-1 in: i) the stromal compartment of axillary lymph nodes of breast cancer patients, to seek a correlation with cancer metastasis; ii) in the stroma of invasive breast carcinomas (IBCs), to confirm data from published studies.
Results
Expression of sCav-1 in lymph nodes
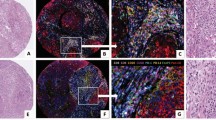
We evaluated the expression of sCav-1 in the stromal compartment of lymph nodes (see Fig. 1) which is composed of: (i) fibroblast-like stromal cells, a heterogenous population that looks like fibroblasts (with spindle shape cytoplasm and elongated nucleus) which encompasses marginal reticular cells under the subcapsular sinus and fibroblastic reticular cells in the paracortex/T cell zone; (ii) follicular dendritic cells in lymphoid follicles (with several cytoplasmic extensions/dendrites); (iii) endothelial cells and (iv) pericytes. These cells have distinctive locations and different functions, some of which not yet fully elucidated.
Illustration of fibroblast-like stromal cells in a lymph node. In the insets three examples of sCav-1 positive cells in a normal lymph node. Together with endothelial cells (black arrow) and pericytes, fibroblast-like stromal cells, such as marginal reticular cells (white arrow) under the subcapsular sinus, fibroblastic reticular cells in the paracortex/T cell zone (*), and follicular dendritic cells in lymphoid follicles (arrowhead) constitute the stromal compartment of a lymph node. All IHC images magnification: 200x. Created with BioRender.com.
According to our knowledge, to date a specific immunomarker for each fibroblast-like stromal cells is lacking, and their identification mostly relies on location and morphologic features by which they can be easily distinguished from lymphocytic cells, plasma cells and monocytes/macrophages.
Among axillary lymph nodes of IBC cases, 119 (65.4%) showed expression of sCav-1 in fibroblast-like stromal cells scattered within lymphoid tissue (see Fig. 2d,e,f and Table 1 for details).
Semiquantitative score of sCav-1 expression. (a) Absent (Score 0), (b) weak (Score 1) and (c) strong (Score 2) expression of sCav-1 in cancer associated fibroblasts (arrowhead) in primary invasive breast cancer. (d) Absent (Score 0), (e) weak (Score 1) and (f) strong (Score 2) expression of sCav-1 in fibroblast-like stromal cells (arrowhead) within metastatic lymph nodes; in the insets Pan-CK expression in metastatic cells. Internal positive controls: endothelial cells (black arrow) and myoepithelial cells (white arrow). All images magnification: ×200.
All normal/reactive axillary lymph nodes of patients included as negative controls (NC) were positive: 17 with score 1 and 3 with score 2 (see Fig. 3b). Instead, a large number of metastatic axillary lymph nodes (Ln +) (26 out of 54, 48%) showed a loss of sCav-1 expression: sCav-1 was significantly less expressed in metastatic axillary lymph nodes (Ln +) than in normal/reactive axillary lymph nodes (Ln−) (p-value = 0.0042) (see Fig. 3a for details). In particular, the difference was strikingly more evident between metastatic axillary lymph nodes (Ln +) and normal/reactive axillary lymph nodes of tumors limited to the breast (Ln−/IBCN−) (p-value < 0.0001); normal/reactive axillary lymph nodes of tumors with axillary metastatic spread (Ln−/IBCN +) showed an in-between sCav-1 profile: higher if compared to metastatic axillary lymph nodes (Ln +) (p-value = 0.0001) and lower if compared, instead, to normal/reactive axillary lymph nodes of tumors limited to the breast (Ln−/IBCN−) (p-value < 0.0001) (see Fig. 3b). Axillary lymph nodes of NC showed a sCav-1 expression higher than normal/reactive axillary lymph nodes of tumors with axillary metastatic spread (Ln−/IBCN +) (p-value = 0.0004) and slightly lower than normal/reactive axillary lymph nodes of tumors limited to the breast (Ln−/IBCN−) (p-value = 0.0305, Fig. 3b). These results indicate that axillary lymph nodes express high levels of sCav-1, but metastatic axillary lymph nodes (Ln +) express lower levels of sCav-1 compared to normal/reactive axillary lymph nodes (Ln−); intriguingly, among normal/reactive axillary lymph nodes those close to metastatic lymph nodes (Ln−/IBCN +) express lower levels of sCav-1 if compared to those from tumors limited to the breast (Ln−/IBCN−).
(a) Expression of sCav-1 in primary IBCs and lymph nodes. In contrast to primary IBCs (regardless the nodal status), a high number of metastatic lymph nodes (Ln +) showed score 0 staining for sCav-1. Ln + demonstrate significant lower expression of sCav-1 than lymph nodes without metastasis (Ln−). (b) sCav-1 expression in lymph nodes. From right to left, sCav-1 is increasingly lost from lymph nodes with reactive hyperplasia from cases without nodal metastatic disease (Ln−/IBCN−), through non-metastatic lymph nodes from cases with nodal metastatic disease (Ln−/IBCN +), to Ln + ; lymph nodes of patients with no history of cancer (NC) show undoubtedly high expression of sCav-1, comparable to Ln−/IBCN−, even if slightly lower. (c) Correlation of sCav-1 expression in primary IBCs and nodal status. The histograms show how primary tumors with nodal metastasis (IBCN +) demonstrate lower expression of sCav-1 than primary tumors without metastasis (IBCN−), regardless the grade. (d) Correlation of sCav-1 expression in primary poorly differentiated (p.d.) IBCs of no special type (NST) and nodal status. The histograms show how IBCN + demonstrate statistically significant lower expression of sCav-1 than IBCN− in the NST p.d. subgroup. sCav-1 staining was scored semi-quantitatively as 0, 1 and 2 (see text).
Expression of sCav-1 in primary breast tumors
sCav-1 expression has been evaluated in all the 189 primary IBC cases examined. Myoepithelial cells and endothelium exhibited strong staining in all the samples examined, as expected, indicating a proper immunohistochemical reaction (see Fig. 2a–c).
In particular, 181 primary tumors (95.8%) [of which 134 cases were IBCs of No Special Type (IBCs-NST) (95.7%) and 47 cases were Invasive Lobular Cancers (ILCs) (95.9%)] stained positive for sCav-1 (67 with score 2, 114 with score 1). 8 cases (4.2%) were negative (score 0).
Of the 189 cases, sCav-1 expression was lower in those tumors with axillary metastatic spread (IBCN +) compared to those limited to the breast (IBCN−), with a p-value close to statistical significance (p = 0.0610, see Fig. 3c).
In the attempt to stratify sCav-1 expression among the different subtypes, we have found: i) no significant difference between IBCs-NST and ILCs; ii) among IBCs-NST, a statistically significant correlation between loss of sCav-1 and presence of lymph node metastases in poorly differentiated carcinomas (p-value = 0.03, see Fig. 3d).
Recurrence rate and sCav-1 expression in primary IBCs
We have collected patients’ clinical data up until 20th of September 2020 (see Supplementary Table S1). According to these data: (i) 28 patients have been lost to follow-up (the loss to follow-up rate was quite low: ~ 15%); (ii) 13 patients with IBC-NST and 7 patients with ILC have relapsed; the total recurrence rate was 13% and, in particular: 12% for IBC NST and 16% for IBC ILC.
Interestingly, even if only ~ 4% of primary IBCs totally lacked the expression of sCav-1 (score 0), we have found that ~ 74% of the relapsed cases had only a weak expression of sCav-1 (score 1) regardless the histotype (10 of 13 were IBC-NST and 5 of 7 were ILC) (see Supplementary Table S2).
In addition, all relapsed cases were grade 3 or 2; none grade 1 tumors relapsed.
In conclusion, our data are in line with the literature; indeed, sCav-1 loss in primary breast cancer is a negative prognostic factor and is common in high grade breast cancer19.
Potential pitfalls
Selected samples represented a relatively small consecutively series in a mono-institutional setting; this could represent a limitation to draw statistically significant conclusions about the correlation between sCav-1 expression and patients outcomes, even if this is not the main aim of the present article. Moreover, this could be the reason for the lower rate of primary IBC sCav-1 negative in respect to the literature18.
This research article would present preliminary results that support new insights on the potential role of sCav-1 in lymph node metastatic niche, paving the way for further investigations. However, data need to be validated in a wide well-studied and well-annotated multicentric cohort with a long term follow-up, in order to confirm envisaged scenarios.
Discussion
To date, the relationship between Cav-1 and cancer remains controversial11. In the past, human studies extensively focused on the role of Cav-1 in epithelial cancer cells reporting a significant increase in Cav-1 expression in malignant lesions than benign breast tissue21,22,23,24,25. In recent decades, increasing attention has been paid to the expression of Cav-1 in the tumor stroma where its loss predicts poor outcome15,16,17. In the present study, immunohistochemistry performed on IBCs primary tumors demonstrated a considerable trend toward significance between a loss of sCav-1 and presence of lymph node metastases. Nevertheless, statistical significance is achieved when only poorly differentiated NST IBCs are considered. These data are in accordance to those from the literature, where a strong association was observed between a loss of sCav-1 and the presence of lymph node metastases in triple negative tumors19. This suggests that especially IBCs with aggressive behavior may be sensitive to sCav-1 regulation in their progression into metastatic disease. In contrast, at present there is still little knowledge regarding the expression of sCav-1 at metastatic sites. Here, DTCs survive and grow as metastasis in presence of a supportive tumor stroma, the so-called “metastatic niche”, whose evolution thus recapitulates stromal progression in the primary tumor5. The formation of the metastatic niche can also be remotely induced by primary tumors before the arrival and establishment of DTCs: for such cases, the term “pre-metastatic niche” has been coined26. This phenomenon has been described also for lymph nodes, where fibroblast-like stromal cells, together with bone marrow derived cells, play a crucial role to form these permissive compartments6. The present study investigated the expression of sCav-1 in the tumor stroma of IBCs and in their corresponding axillary lymph nodes, in order to define any possible correlation with cancer metastasis. In accordance to Alevizos et al., sCav-1 was significantly less expressed in lymph node metastases than lymph nodes with reactive hyperplasia, of metastatic or non-metastatic cases, confirming that low expression of Cav-1 in fibroblast-like stromal cells of axillary lymph nodes correlates with high metastatic potential27. Intriguingly, Celus et al. recently demonstrated in mouse models of lung and breast cancer metastasis that Cav-1 inhibition in the so-called metastasis-associated macrophages (MAMs) drives metastatic growth favoring angiogenesis by the restriction of vascular endothelial growth factor A/vascular endothelial growth factor receptor 1 (VEGF-A/VEGFR1) signaling and its downstream effectors at the metastatic site28. A similar mechanism may be involved in humans. For the first time to our best knowledge, we described a statistically significant progressive loss of sCav-1 from normal/reactive axillary lymph nodes from tumors limited to the breast (Ln−/IBCN−) to metastatic axillary lymph nodes (Ln +), through normal/reactive axillary lymph nodes of tumors with axillary metastatic spread (Ln−/IBCN +). These data may suggest that Cav-1 expressed by the stromal compartment of lymph nodes, somehow, may play a possible role in establishing the pre-metastatic niche in IBC. The lower expression of sCav-1, observed not only in lymph node metastases but also in close lymph nodes with reactive hyperplasia (Ln−/IBCN +), may hint at the possible role of the primary tumor or of the DTCs in the microenvironmental changes of loco-regional lymph nodes, contributing to the formation of these permissive compartments. In order to better investigate which of the two—the primary tumor or DTCs—were more likely to be involved, we included lymph nodes from patients with no history of cancer but with reactive hyperplasia (as negative control, NC), which showed consistently high expression of sCav-1. This would hint at the possibility that sCav-1 in lymph nodes might be modulated by DTCs, present in the same lymph node or in close vicinity, and not remotely by primary tumors at distance. Indeed, if the primary tumor exerted a permissive effect for the infiltration and growth of DTCs in the axilla, lymph nodes from patients with tumors limited to the breast (Ln−/IBCN−) should have shown a lower expression of sCav-1 than NC. Instead, the expression of sCav-1 in NC resulted even lower than axillary lymph nodes from patients with tumors limited to the breast (Ln−/IBCN−). According to this hypothesis, DTCs might in turn provide the permissive pre-metastatic niche in close lymph nodes. In 2005, Kaplan et al. firstly demonstrated that bone marrow-derived hematopoietic progenitor cells that express vascular endothelial growth factor receptor 1 (VEGFR1) home to tumor-specific pre-metastatic sites and form cellular clusters before the arrival of tumor cells6. The authors also showed that VEGFR1 + cells express VLA-4 (also known as integrin α4β1), and that tumor-specific growth factors upregulate fibronectin—a VLA-4 ligand—in resident fibroblasts, providing a permissive niche for incoming tumor cells. According to this, recent evidence suggest that tumor secreted factors modify perivascular cells to create a pro-metastatic environment enriched in fibronectin29. In 2008, Shi and Sottile showed how Cav-1-dependent β1 integrin endocytosis is a critical regulator of fibronectin turnover; in particular, they demonstrated that Cav-1 downregulation reduces both fibronectin and β1 integrin endocytosis30. Within this scenario, a loss of expression of sCav-1 in lymph nodes may contribute to the localized deposition of fibronectin, providing permissive compartments for DTCs. In addition, sCav-1 has been recently described to promote the ability of dendritic cells (DC) to reach the lymph nodes and initiate CD8 + T cells response. Indeed, Oyarce et al. have reported that caveolin-1 expression increases upon maturation in DCs and promotes their migration to lymph nodes favoring the activation of CD8 + T cells. CD8 + T cells response represents a key mechanism against pathogens but also against solid tumors. To confirm this hypothesis the Authors demonstrated a reduction in CD8 + T cell response and antitumor protection in mice that received CAV−/− DCs31. These data strongly suggest that a loss of expression of sCav-1 in lymph nodes may inhibit anti-tumor CD8 + T cells, thus favoring the initiating capacity of incoming DTCs. Finally, immunohistochemical studies of human breast cancer showed that specimens lacking sCav-1 overexpress key enzymes of the glycolytic pathway, such as PKM2 and LDH32,33, thus generating, in the pre-metastatic niche, high quantities of energy metabolites (i.e. lactate and pyruvate) to feed the arriving DTCs. As a result, the loss of expression of sCav-1 in axillary lymph nodes may promote successful seeding of arriving cancer cells via at least three fascinating mechanisms: the increase of fibronectin deposition, the inhibition of anti-tumor CD8 + T cells and the increase of energy metabolites availability. Further studies are needed to better understand either the mechanisms through which a loss of sCav-1 may contribute to the regulation of metastatic niche formation or the mechanisms through which cancer cells induce the downregulation of sCav-1, a process currently still largely poorly understood.
In conclusion, our findings, in addition to supporting data from the literature, provide further and new evidence of a correlation between sCav-1 expression in lymph nodes and lymph node metastases, suggesting a possible role in the establishment of the pre-metastatic niche in breast cancer.
Further analyses on larger cohorts of patients with adequate number of lymph node metastases are required to validate these preliminary data definitely; moreover, functional studies in vitro are needed to study in depth the biological mechanisms underlying these preliminary observations.
Materials and methods
Case selection
189 cases (53 with lymph node metastases and 136 without) were examined, from women with a median age of 57.55 years (range 31–89 years). All IBCs were classified according to 2019 World Health Organization (WHO) Classification into IBC-NST, graded according to the Nottingham Modification of the Bloom-Richardson system, and ILC (Table 2).
137 axillary lymph nodes were collected from 28 cases with axillary metastatic spread (IBCN +); among these, 54 displayed metastasis (Ln +), 83 did not (Ln−/IBCN +). In addition, 45 axillary lymph nodes were collected from other 28 cases with tumors limited to the breast (Ln−/IBCN−). Therefore, three groups of study interest were delineated (Table 3): (i) metastatic axillary lymph nodes (Ln +); (ii) normal/reactive axillary lymph nodes of tumors with axillary metastatic spread (Ln−/IBCN +); (iii) normal/reactive axillary lymph nodes of tumors limited to the breast (Ln−/IBCN−).
Twenty normal/reactive axillary lymph nodes of patients admitted at our hospital for cardiac surgeries and with no history of cancer were included as negative controls (NC). All of the specimens studied were retrieved from the files of the Division of Surgical, Molecular, and Ultrastructural Pathology, University of Pisa (Italy) from January 2006 to January 2011.
sCav-1 was evaluated: (i) in lymph nodes, in fibroblast-like stromal cells classified as marginal reticular cells (below the sub-capsular sinus), fibroblastic reticular cells (of the paracortex/T cell zone) and follicular dendritic cells (of B follicles) (see Fig. 1); (ii) in IBCs, in fibroblasts scattered in the tumor stroma.
All investigations were conducted according to the principles expressed in the Declaration of Helsinki; this study was approved by the "Ethical Committee for the testing and evaluation of clinical study protocols in the North Wester Tuscany Area (CEAVNO)" (Prot. n: 14,835. Approval date: 11/04/2019). Written informed consent was obtained from each participant.
Immunohistochemistry
Formalin-fixed, paraffin-embedded (FFPE) tissue blocks were cut into 4 µm thick sections. Slides were incubated with rabbit polyclonal anti-Caveolin-1 antibody (N20) (Santa Cruz CA, dilution 1:500) at 37 °C for 32 min (Ventana Benchmark XT staining system, Ventana Medical System-Roche) and developed in diaminobenzidine (DAB)—hydrogen peroxide for 10 min (ultraView Universal DAB kit, Ventana Medical System-Roche). Finally, sections were counterstained with hematoxylin and mounted. The staining was scored semi-quantitatively as 0 (negative, in stromal cells), 1 (diffuse weak or strong in less than 30% of stromal cells) and 2 (strong in 30% or more of the stromal cells)20. Both membranous or combined membranous and cytoplasmic staining was used for Cav-1 expression evaluation. Positive staining of myoepithelial cells or endothelium, known to be abundant in Cav-1, served as an internal positive control.
Statistical analysis
The Chi-Square test was used to analyze the association between the different phenotypes and Cav-1 expression. All statistical analyses were performed by means of Medcalc for Windows (Version 12). A p-value of less than 0.05 was considered statistically significant.
References
Nicolas, E., Bertucci, F., Sabatier, R. & Goncalves, A. Targeting BRCA deficiency in breast cancer: what are the clinical evidences and the next perspectives?. Cancers (Basel). https://doi.org/10.3390/cancers10120506 (2018).
Elsheikh, S. E. et al. Caveolin 1 and Caveolin 2 are associated with breast cancer basal-like and triple-negative immunophenotype. Br. J. Cancer 99, 327–334. https://doi.org/10.1038/sj.bjc.6604463 (2008).
Blasi, F. & Sidenius, N. The urokinase receptor: Focused cell surface proteolysis, cell adhesion and signaling. FEBS Lett. 584, 1923–1930. https://doi.org/10.1016/j.febslet.2009.12.039 (2010).
Kalluri, R. & Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 6, 392–401. https://doi.org/10.1038/nrc1877 (2006).
Sleeman, J. P. The metastatic niche and stromal progression. Cancer Metastasis Rev. 31, 429–440. https://doi.org/10.1007/s10555-012-9373-9 (2012).
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827. https://doi.org/10.1038/nature04186 (2005).
Lisanti, M. P., Scherer, P. E., Tang, Z. & Sargiacomo, M. Caveolae, caveolin and caveolin-rich membrane domains: a signalling hypothesis. Trends Cell Biol. 4, 231–235. https://doi.org/10.1016/0962-8924(94)90114-7 (1994).
Chen, D. & Che, G. Value of caveolin-1 in cancer progression and prognosis: Emphasis on cancer-associated fibroblasts, human cancer cells and mechanism of caveolin-1 expression (review). Oncol. Lett. 8, 1409–1421. https://doi.org/10.3892/ol.2014.2385 (2014).
Williams, T. M. & Lisanti, M. P. The Caveolin genes: From cell biology to medicine. Ann. Med. 36, 584–595. https://doi.org/10.1080/07853890410018899 (2004).
Glenney, J. R. Jr. Tyrosine phosphorylation of a 22-kDa protein is correlated with transformation by Rous sarcoma virus. J. Biol. Chem. 264, 20163–20166 (1989).
Patani, N., Martin, L. A., Reis-Filho, J. S. & Dowsett, M. The role of caveolin-1 in human breast cancer. Breast Cancer Res. Treat. 131, 1–15. https://doi.org/10.1007/s10549-011-1751-4 (2012).
Hayashi, K. et al. Invasion activating caveolin-1 mutation in human scirrhous breast cancers. Cancer Res. 61, 2361–2364 (2001).
Zajchowski, D. A. et al. Identification of gene expression profiles that predict the aggressive behavior of breast cancer cells. Cancer Res. 61, 5168–5178 (2001).
Mercier, I. et al. Human breast cancer-associated fibroblasts (CAFs) show caveolin-1 downregulation and RB tumor suppressor functional inactivation: Implications for the response to hormonal therapy. Cancer Biol. Ther. 7, 1212–1225. https://doi.org/10.4161/cbt.7.8.6220 (2008).
Witkiewicz, A. K. et al. An absence of stromal caveolin-1 expression predicts early tumor recurrence and poor clinical outcome in human breast cancers. Am. J. Pathol. 174, 2023–2034. https://doi.org/10.2353/ajpath.2009.080873 (2009).
Ren, M. et al. Absence of caveolin-1 expression in carcinoma-associated fibroblasts of invasive micropapillary carcinoma of the breast predicts poor patient outcome. Virchows Arch. 465, 291–298. https://doi.org/10.1007/s00428-014-1614-6 (2014).
Eliyatkin, N. et al. Expression of stromal Caveolin-1 may be a predictor for aggressive behaviour of breast cancer. Pathol. Oncol. Res. 24, 59–65. https://doi.org/10.1007/s12253-017-0212-8 (2018).
Sloan, E. K. et al. Stromal cell expression of caveolin-1 predicts outcome in breast cancer. Am. J. Pathol. 174, 2035–2043. https://doi.org/10.2353/ajpath.2009.080924 (2009).
Witkiewicz, A. K. et al. Loss of stromal caveolin-1 expression predicts poor clinical outcome in triple negative and basal-like breast cancers. Cancer Biol. Ther. 10, 135–143. https://doi.org/10.4161/cbt.10.2.11983 (2010).
Witkiewicz, A. K. et al. Stromal caveolin-1 levels predict early DCIS progression to invasive breast cancer. Cancer Biol. Ther. 8, 1071–1079. https://doi.org/10.4161/cbt.8.11.8874 (2009).
Yang, G. et al. Elevated expression of caveolin is associated with prostate and breast cancer. Clin. Cancer Res. 4, 1873–1880 (1998).
Savage, K. et al. Caveolin 1 is overexpressed and amplified in a subset of basal-like and metaplastic breast carcinomas: A morphologic, ultrastructural, immunohistochemical, and in situ hybridization analysis. Clin. Cancer Res. 13, 90–101. https://doi.org/10.1158/1078-0432.CCR-06-1371 (2007).
Pinilla, S. M., Honrado, E., Hardisson, D., Benitez, J. & Palacios, J. Caveolin-1 expression is associated with a basal-like phenotype in sporadic and hereditary breast cancer. Breast Cancer Res. Treat. 99, 85–90. https://doi.org/10.1007/s10549-006-9184-1 (2006).
Liedtke, C., Kersting, C., Burger, H., Kiesel, L. & Wulfing, P. Caveolin-1 expression in benign and malignant lesions of the breast. World J. Surg. Oncol. 5, 110. https://doi.org/10.1186/1477-7819-5-110 (2007).
Hurlstone, A.F. et al. Analysis of the CAVEOLIN-1 gene at human chromosome 7q31.1 in primary tumours and tumour-derived cell lines. Oncogene 18, 1881–1890, https://doi.org/10.1038/sj.onc.1202491 (1999).
Sleeman, J. P. et al. Concepts of metastasis in flux: The stromal progression model. Semin. Cancer Biol. 22, 174–186. https://doi.org/10.1016/j.semcancer.2012.02.007 (2012).
Alevizos, L. et al. Breast cancer nodal metastasis correlates with tumour and lymph node methylation profiles of Caveolin-1 and CXCR4. Clin. Exp. Metastasis 31, 511–520. https://doi.org/10.1007/s10585-014-9645-6 (2014).
Celus, W. et al. Loss of caveolin-1 in metastasis-associated macrophages drives lung metastatic growth through increased angiogenesis. Cell Rep. 21, 2842–2854. https://doi.org/10.1016/j.celrep.2017.11.034 (2017).
Murgai, M. et al. KLF4-dependent perivascular cell plasticity mediates pre-metastatic niche formation and metastasis. Nat. Med. 23, 1176–1190. https://doi.org/10.1038/nm.4400 (2017).
Shi, F. & Sottile, J. Caveolin-1-dependent beta1 integrin endocytosis is a critical regulator of fibronectin turnover. J. Cell Sci. 121, 2360–2371. https://doi.org/10.1242/jcs.014977 (2008).
Oyarce, C. et al. Caveolin-1 expression increases upon maturation in dendritic cells and promotes their migration to lymph nodes thereby favoring the induction of CD8(+) T cell responses. Front. Immunol. 8, 1794. https://doi.org/10.3389/fimmu.2017.01794 (2017).
Witkiewicz, A. K. et al. Molecular profiling of a lethal tumor microenvironment, as defined by stromal caveolin-1 status in breast cancers. Cell Cycle 10, 1794–1809. https://doi.org/10.4161/cc.10.11.15675 (2011).
Pavlides, S. et al. The reverse Warburg effect: Aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 8, 3984–4001. https://doi.org/10.4161/cc.8.23.10238 (2009).
Author information
Authors and Affiliations
Contributions
C.S. and G.F. selected the cases, wrote and submitted the protocol to the ethical committee, analyzed the results, and wrote the manuscript. G.N.F. reviewed the retrieved cases, collected clinical data and created tables and figures. M.M. performed the immunohistochemical staining. P.A. performed statistical analysis. V.O. collected the cases. S.P.C. and L.I. collected the written informed consents. F.S. and M.P.L. conceived the study. A.G.N. conceived and coordinated the study and edited the manuscript. All authors reviewed and approved the current version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Scatena, C., Fanelli, G., Fanelli, G.N. et al. New insights in the expression of stromal caveolin 1 in breast cancer spread to axillary lymph nodes. Sci Rep 11, 2755 (2021). https://doi.org/10.1038/s41598-021-82405-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-82405-y
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.