Abstract
Podoplanin, which is immunoreactive to D2-40 antibody, is reportedly expressed in lymphatic vessels in non-neoplastic tissues, and also in vascular and non-vascular tumors. However, its expression in non-neoplastic and neoplastic liver tissues has not been well documented. In this study, we examined podoplanin expression in specimens from 10 normal livers and 73 cases of liver tumors: hemangioma (16 cases), epithelioid hemangioendothelioma (9 cases), angiosarcoma (4 cases), angiomyolipoma (7 cases), hepatocellular carcinoma (11 cases), intrahepatic cholangiocarcinoma (11 cases), and metastatic liver cancer (15 cases). We compared levels of podoplanin and other endothelial markers (CD31, CD34, and factor VIII) in liver tumors. In the normal liver, podoplanin was expressed in lymphatic endothelium, nerve fibers, and mesothelium in the hepatic capsule, but not observed in any cells within hepatic lobules. Among liver tumors, podoplanin was specifically expressed in seven of nine cases (78%) of epithelioid hemangioendothelioma but not in other hepatic tumors. The expression of CD31, CD34, and factor VIII was observed in endothelial cells in all cases of hemangioma, epithelioid hemangioendothelioma, angiosarcoma, and angiomyolipoma with one exception, a case of epithelioid hemangioendothelioma which was without CD31 expression. Interestingly, the intensity of podoplanin expression was negatively correlated with the expression of CD34 and factor VIII. In conclusion, podoplanin would be useful as a diagnostic marker for epithelioid hemangioendothelioma in liver tumors.
Similar content being viewed by others
Main
The D2-40 monoclonal antibody was first reported to recognize an oncofetal antigen (M2A) expressed in normal testis as well as testicular germ cell tumors.1 M2A is an almost 40 kDa O-linked sialoglycoprotein expressed at the cell surface.1 Recently, this antigen was shown to be the same as a lymphatic marker, podoplanin (E11, T1A-2, Aggrus), which was specifically expressed on lymphatic but not vascular endothelial cells.2 Distinguishing between lymphatic vessels and blood capillaries is difficult using only hematoxylin and eosin (H&E) staining, and a commercially available D2-40 antibody reactive to podoplanin has been used to evaluate the lymphatic invasion of cancer in pathological examinations.3 In addition, podoplanin was reported to be a useful marker for the diagnosis of vascular and non-vascular tumors of various organs.4 Podoplanin was expressed by some vascular tumors with lymphatic differentiation, such as lymphangioma, Kaposi's sarcoma, Kaposiform hemangioendothelioma (so-called Dabska tumor), hobnail hemangioma, and a subset of angiosarcomas.5, 6, 7, 8, 9, 10 However, the expression of podoplanin in liver tumors has not been well documented.
In this study, we examined the expression of podoplanin in normal liver tissues, primary liver tumors of vascular or non-vascular origin, and metastatic liver cancers, and compared its diagnostic usefulness with other endothelial markers (CD31, CD34, and factor VIII).5
Materials and methods
Case Selection and Tissue Preparation
Specimens from 58 cases of primary liver tumor, 15 cases of metastatic liver tumor, and 10 normal livers were studied. The primary liver tumors included hemangioma (16 cases), epithelioid hemangioendothelioma (9 cases), angiosarcoma (4 cases), angiomyolipoma (7 cases), hepatocellular carcinoma (11 cases), and intrahepatic cholangiocarcinoma (11 cases). We used biopsied, surgically resected, necropsied, or autopsied specimens. Normal liver specimens were obtained by surgical resection for metastatic liver tumors. Average ages, male/female ratios, and types of specimens of the examined cases are shown in Table 1.
Of the 16 hemangiomas, 15 were cavernous. The remaining tumor was a sclerosing hemangioma containing several neoplastic cells at its periphery. Two cases of epithelioid hemangioendothelioma involved a solitary lesion (2.0 and 3.0 cm in diameter, respectively), whereas in seven cases there were multiple lesions (three cases, two lesions; four cases, more than two lesions). In addition, in one case, cutaneous metastasis was detected in the right chest. A histological specimen obtained from the cutaneous metastasis was also used in this study. All cases of epithelioid hemangioendothelioma were histologically characterized by the proliferation of epithelioid endothelial cells in the fibrous and myxoid stroma. Intracellular lumina (intracytoplasmic vacuoles) were easily found. Tumor cells showed mild to moderate cellular atypia, although high-grade cellular atypia corresponding to angiosarcoma was not observed in any cases of epithelioid hemangioendothelioma. Out of the four cases of angiosarcoma, one patient had a history of Thorotrast injections, and another had a history of working with vinyl-chloride monomer. All angiosarcomas diffusely involved the liver. One patient with angiomyolipoma had Bourneville–Pringle disease, and also had an angiomyolipoma in the left kidney. All cases of hepatocellular carcinoma involved chronic advanced liver disease in the background. Nine patients had chronic viral hepatitis (three HBV-related and six HCV-related cases), and two had alcoholic liver disease.
We also used 15 cases of metastatic liver tumor from the colorectum (eight cases), stomach (four cases), pancreas (one case), breast (one case), and kidney (one case). We selected moderately to poorly differentiated adenocarcinoma (14 cases) and grade 3 renal cell carcinoma (one case), because the morphologic differential diagnosis of epithelioid hemangioendothelioma typically is with poorly differentiated adenocarcinoma.
Specimens were fixed in 10% formalin, embedded in paraffin, and then cut into sections 4 μm thick for H&E staining and for immunohistochemistry.
Immunohistochemistry
Immunostaining of podoplanin, CD31, CD34, and factor VIII was performed using the Envision+system (Dako Cytomation, Glostrup, Denmark). The primary antibodies used were anti-podoplanin (clone D2-40; 1:100 dilution; Dako Cytomation), anti-CD31 (clone JC70A; 1:40; Dako Cytomation), anti-CD34 (clone QBEND 10; 1:200; Immunotech, Marseille, France), and anti-factor VIII (clone F8/86; 1:200; Dako Cytomation). Before incubation with each of the primary antibodies, the sections for podoplanin, CD31, and factor VIII were microwaved in citrate buffer (pH 6.0) for 20 min. Negative controls were evaluated by substituting the primary antibody with similarly diluted non-immunized mouse serum.
The expression of podoplanin, CD31, CD34, and factor VIII in epithelioid hemangioendothelioma cases was evaluated semiquantitatively using 4 scores, according to the percentage of positive cells in individual lesions: − (negative), 0%; 1+ (focal), 1–10%; 2+ (moderate), 11–50%; 3+ (extensive), more than 50%.
Statistical Analysis
Differences between the two groups were analyzed using the Mann–Whitney U-test. The correlation in intensity of immunohistochemical expression among antibodies was analyzed with the Spearman's correlation coefficient by rank test. A P-value less than 0.05 was considered statistically significant.
Results
Expression of Podoplanin and Other Endothelial Markers in the Normal Liver
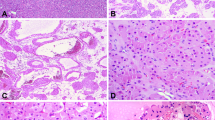
In normal liver, podoplanin was expressed by lymphatic but not vascular or sinusoidal endothelial cells (Figure 1). Lymph vessels were distributed in all small to large portal tracts in the liver and always accompanied by a portal triad (bile ducts, hepatic arteries, and portal veins). Lymph vessels were usually smaller than portal veins, and appeared as slit-like canals. Several lymphocytes were sometimes found in these canals. There were no cells positive for podoplanin in the hepatic parenchyma. Other components expressing podoplanin were nerve fibers in the hepatic hilum and mesothelial cells covering the hepatic capsule. Spindle-shaped mesenchymal cells surrounding intrahepatic large bile ducts were weakly positive for podoplanin.
Expression of podoplanin and CD34 in the normal liver. Podoplanin is specifically expressed in lymphatic endothelial cells. CD34 is detected in both lymphatic and vascular endothelial cells. Some sinusoidal endothelial cells surrounding portal tracts are focally positive for CD34 (all images, × 400 in almost the same field).
Endothelial cells of both lymphatic and blood vessels were positive for CD31, CD34, and factor VIII (Figure 1). Sinusoidal endothelial cells were negative for factor VIII but focally and weakly positive for CD31 or CD34. Hepatocytes and biliary epithelial cells were completely negative for podoplanin, CD31, CD34, and factor VIII.
Expression of Podoplanin and Other Endothelial Markers in Liver Tumors
The expression of podoplanin and other endothelial markers is summarized in Table 2. Interestingly, podoplanin was expressed in seven of nine cases (78%) of epithelioid hemangioendothelioma (Figure 2). Podoplanin expression in epithelioid hemangioendothelioma was extensive (3+) in four cases, moderate (2+) in two cases, and focal (1+) in one case. Podoplanin was observed on the cellular membrane of neoplastic cells. Same as in the primary tumor of the epithelioid hemangioendothelioma case, neoplastic cells in the cutaneous metastasis were positive for podoplanin. In contrast, other tumors including hemangioma, angiosarcoma, angiomyolipoma, hepatocellular carcinoma, intrahepatic cholangiocarcinoma, and metastatic liver cancer were all negative for podoplanin. Podoplanin expression was significantly greater in epithelioid hemangioendothelioma than hemangioma (P<0.01), angiosarcoma (P<0.05), angiomyolipoma (P<0.01), hepatocellular carcinoma (P<0.01), intrahepatic cholangiocarcinoma (P<0.01), and metastatic liver cancer (P<0.01) (Table 2).
Expression of podoplanin and CD34 in primary liver tumors. In epithelioid hemangioendothelioma, epithelioid tumor cells are diffusely positive for podoplanin and CD34. Podoplanin is mainly expressed on the cellular membrane (inset). The expression of podoplanin is intense in the area in which the expression of CD34 is weak (left lower area). In hemangioma, podoplanin is expressed in lymphatic vessels in the fibrous septa. CD34 is expressed in both lymphatic vessels and neoplastic endothelial cells. In angiosarcoma and angiomyolipoma, neoplastic endothelial cells are positive for CD34, although podoplanin expression is not observed (all images, × 100; inset, × 400; three images of epithelioid hemangioendothelioma in almost the same field).
In the cases of cavernous hemangioma, lymphatic vessels positive for podoplanin were observed in the fibrous stroma. These vessels were much smaller than cavernous neoplastic vessels and sometimes contained small lymphocytes. Neoplastic endothelial cells of cavernous vessels were always negative for podoplanin, and there was no continuity between podoplanin-negative cavernous vessels and podoplanin-positive lymphatic vessels (Figure 2). In the cases of angiosarcoma and angiomyolipoma, podoplanin was not expressed in any tumor cells (Figure 2). In the cases of hepatocellular carcinoma, podoplanin was expressed in lymphatic vessels in the tumor capsules and fibrous septa. Spindle-shaped mesenchymal cells embedded in tumor capsules were weakly and focally positive for podoplanin. In the cases of intrahepatic cholangiocarcinoma and metastatic liver cancer, the expression of podoplanin was observed in lymphatic vessels and mesenchymal cells in the fibrous stroma. CD31, CD34, and factor VIII were always expressed by neoplastic endothelial cells in cases of epithelioid hemangioendothelioma, hemangioma, angiosarcoma, and angiomyolipoma with one exception, a case of epithelioid hemangioendothelioma in which CD31 was not expressed (Figure 2). The expression of CD31, CD34, and factor VIII was significantly increased in vascular tumors compared to non-vascular tumors (P<0.01), although there was no difference among vascular tumors (Table 2).
Diagnostic Usefulness of Podoplanin for Epithelioid Hemangioendothelioma
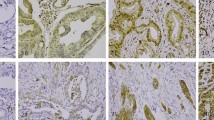
Among vascular tumors including hemangioma, epithelioid hemangioendothelioma, and angiosarcoma, the sensitivity and specificity of podoplanin expression for the diagnosis of epithelioid hemangioendothelioma were 78 and 100%, respectively. The expression of podoplanin, CD31, CD34, and factor VIII is summarized in Table 3. Interestingly, the three tumors diffusely positive for podoplanin were also diffusely positive for CD31, but only focally positive for CD34 and factor VIII (Figure 3). In contrast, the two patients negative for podoplanin showed no or focal expression of CD31, and were diffusely positive for CD34 and factor VIII (Figure 3). The expression of podoplanin was correlated positively with that of CD31 (r=0.793, P=0.02), and negatively with that of CD34 and factor VIII (r=−0.694, P<0.01).
Expression of podoplanin, CD31, CD34, and factor VIII in two representative cases of epithelioid hemangioendothelioma. The upper panel (case 1 in Table 3) shows diffuse expression of podoplanin and CD31, and no expression of CD34 and factor VIII. The lower panel (case 8 in Table 3) does not show the expression of podoplanin and CD31, but CD34 and factor VIII are diffusely expressed in this case (all images, × 400).
Discussion
The results of the present study can be summarized as follows: (1) in normal liver, podoplanin was specifically expressed by lymphatic but not vascular endothelial cells; (2) podoplanin was expressed in epithelioid hemangioendothelioma with 78% sensitivity and 100% specificity among primary and metastatic liver tumors; (3) in cases of epithelioid hemangioendothelioma, podoplanin expression was positively correlated with CD31 expression, and negatively correlated with the expression of CD34 and factor VIII.
Epithelioid hemangioendothelioma is a rare neoplasm of vascular origin that involves soft-tissue and visceral organs such as the liver and lung. The term epithelioid hemangioendothelioma was established by Drs Weiss and Enzinger to describe a soft-tissue tumor of endothelial origin with a clinical course in between that of benign hemangioma and that of angiosarcoma.11 Primary hepatic epithelioid hemangioendothelioma, first reported by Ishak et al,12 shows low-grade malignant potential and has a better clinical outcome than angiosarcoma.13 It is important to distinguish epithelioid hemangioendothelioma from carcinoma or angiosarcoma, because patients with epithelioid hemangioendothelioma can survive for a long term. A histological diagnosis of hepatic epithelioid hemangioendothelioma is sometimes difficult, especially by needle biopsy. Immunostaining of cytokeratin and endothelial markers like CD31, CD34, and factor VIII is usually used to differentiate epithelioid hemangioendothelioma from carcinomas including metastatic carcinomas. As we showed in this study, the expression of podoplanin was negatively correlated with the expression of CD34 and factor VIII. Adding podoplanin to the panel of endothelial markers could improve diagnostic accuracy.
The expression of podoplanin detected by the D2-40 monoclonal antibody can be used to clearly differentiate lymphatic and vascular endothelial cells in normal livers.8 Lymphatic vessels were distributed in all small to large portal tracts, but were not observed in hepatic parenchyma. This result might give insight into the lymphangiogenesis during normal liver development.14, 15, 16 We also detected podoplanin in nerve fascicles, mesothelium covering the liver surface, and stromal cells around the intrahepatic large bile ducts but not in biliary epithelial cells. Schacht et al,2 detected podoplanin in biliary epithelial cells of normal liver using tissue microarrays. Further analyses at both the mRNA and protein levels seem mandatory to resolve this discrepancy.
Kahn et al10 and Fukunaga9 examined podoplanin expression in vascular tumors using D2-40. Cases of epithelioid hemangioendothelioma were included in both studies. Kahn et al10 examined one case of epithelioid hemangioendothelioma that was not reactive with D2-40. Fukunaga9 used 10 cases of epithelioid hemangioendothelioma, and concluded that one of them was reactive to D2-40. Although the cases used in their studies were not site-specified, podoplanin seems to be more commonly expressed in hepatic epithelioid hemangioendothelioma. Kahn et al10 also reported that three of seven angiosarcomas were reactive to D2-40, and angiosarcoma could be divided into two subsets: D2-40-positive/CD31-positive and D2-40-negative/CD31-positive. They speculated that the former subset originated from progenitor cells capable of differentiating along both the lymphatic and blood vessel lineages, whereas the latter subset might be derived from cells restricted to differentiating along the blood vessel lineage. On the basis of their hypothesis, hepatic epithelioid hemangioendothelioma might more commonly originate from progenitor cells capable of differentiating along both the lymphatic and blood vessel lineages.
Previous reports showed that in several cases of extrahepatic angiosarcoma, podoplanin was expressed,6, 9, 10 although in no cases of hepatic angiosarcoma was podoplanin expressed in this study. Four cases might be too small of a sample to conclude that primary hepatic angiosarcomas do not express podoplanin, and further studies using more cases of angiosarcoma arising in liver are needed.
In conclusion, podoplanin was specifically expressed in epithelioid hemangioendothelioma among primary and metastatic liver tumors. Podoplanin could be useful for the diagnosis of hepatic epithelioid hemangioendothelioma.
References
Marks A, Sutherland DR, Bailey D, et al. Characterization and distribution of an oncofetal antigen (M2A antigen) expressed on testicular germ cell tumours. Br J Cancer 1999;80:569–578.
Schacht V, Dadras SS, Johnson LA, et al. Up-regulation of the lymphatic marker podoplanin, a mucin-type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors. Am J Pathol 2005;166:913–921.
Van den Eynden GG, Van der Auwera I, Van Laere SJ, et al. Distinguishing blood and lymph vessel invasion in breast cancer: a prospective immunohistochemical study. Br J Cancer 2006;94:1643–1649.
Ordóñez NG . Podoplanin: a novel diagnostic immunohistochemical marker. Adv Anat Pathol 2006;13:83–88.
Arai E, Kuramochi A, Tsuchida T, et al. Usefulness of D2-40 immunohistochemistry for differentiation between kaposiform hemangioendothelioma and tufted angioma. J Cutan Pathol 2006;33:492–497.
Breiteneder-Geleff S, Soleiman A, Kowalski H, et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol 1999;154:385–394.
Debelenko LV, Perez-Atayde AR, Mulliken JB, et al. D2-40 immunohistochemical analysis of pediatric vascular tumors reveals positivity in kaposiform hemangioendothelioma. Mod Pathol 2005;18:1454–1460.
Franke FE, Steger K, Marks A, et al. Hobnail hemangiomas (targetoid hemosiderotic hemangiomas) are true lymphangiomas. J Cutan Pathol 2004;31:362–367.
Fukunaga M . Expression of D2-40 in lymphatic endothelium of normal tissues and in vascular tumours. Histopathology 2005;46:396–402.
Kahn HJ, Bailey D, Marks A . Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi's sarcoma and a subset of angiosarcomas. Mod Pathol 2002;15:434–440.
Weiss SW, Enzinger FM . Epithelioid hemangioendothelioma: a vascular tumor often mistaken for a carcinoma. Cancer 1982;50:970–981.
Ishak KG, Sesterhenn IA, Goodman ZD, et al. Epithelioid hemangioendothelioma of the liver: a clinicopathologic and follow-up study of 32 cases. Hum Pathol 1984;15:839–852.
Terada T, Nakanuma Y, Hoso M, et al. Hepatic epithelioid hemangioendothelioma in primary biliary cirrhosis. Gastroenterology 1989;97:810–811.
Ishikawa Y, Akasaka Y, Kiguchi H, et al. The human renal lymphatics under normal and pathological conditions. Histopathology 2006;49:265–273.
Hong YK, Harvey N, Noh YH, et al. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn 2002;225:351–357.
Kim KE, Sung HK, Koh GY . Lymphatic development in mouse small intestine. Dev Dyn 2007;236:2020–2025.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fujii, T., Zen, Y., Sato, Y. et al. Podoplanin is a useful diagnostic marker for epithelioid hemangioendothelioma of the liver. Mod Pathol 21, 125–130 (2008). https://doi.org/10.1038/modpathol.3800986
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800986
Keywords
This article is cited by
-
Selection of treatment for hepatic epithelioid hemangioendothelioma: a single-center experience
World Journal of Surgical Oncology (2019)
-
Primary Liver Tumors Other than Hepatocellular Carcinoma: Clinical and Molecular Pearls
Current Hepatology Reports (2018)
-
A rare case of intravascular epithelioid hemangioendothelioma of the cephalic vein treated with surgery and postoperative radiation therapy: a case report and review of the literature
Journal of Medical Case Reports (2015)
-
Lymphatic vessels: new targets for the treatment of inflammatory diseases
Angiogenesis (2014)