Abstract
Epithelioid hemangioendothelioma (EHE) with YAP1-TFE3 fusion is a recently characterized distinctive variant of EHE that accounts for a small subset (<5%) of cases. It is composed of nests of epithelioid cells with voluminous pale cytoplasm and often shows focally vasoformative architecture. TFE3 immunohistochemistry (IHC) can be used to support the diagnosis; however, studies have questioned its specificity. Yes-associated protein 1 (YAP1), part of the Hippo signaling pathway, is expressed in normal endothelial cells, but becomes disrupted in EHE variant with YAP1-TFE3, such that only a small N-terminal region of YAP1 is expressed in the fusion protein. A recent study also reported YAP1 rearrangements in a subset of retiform and composite hemangioendotheliomas (RHE and CHE). In this study, we evaluated the diagnostic utility of an antibody directed against the C-terminus of YAP1 (YAP1-CT) for EHE with YAP1-TFE3, RHE, and CHE. In total, 78 tumors were included in the study: EHE variant with YAP1-TFE3 (n = 13), conventional (CAMTA1-positive) EHE (n = 20), pseudomyogenic hemangioendothelioma (n = 10), epithelioid hemangioma (n = 19), epithelioid angiosarcoma (n = 10), RHE (n = 4), and CHE (n = 2). IHC was performed using a rabbit monoclonal anti-YAP1 C-terminus antibody. EHE variant showed complete loss of YAP1-CT expression in 10 of 13 (77%) cases. All cases of RHE and CHE, with previously confirmed YAP1 rearrangements, also showed loss of YAP1-CT expression. Loss of YAP1-CT was seen in one conventional EHE (1/20; 5%). All other epithelioid vascular tumors showed retained YAP1-CT expression. Loss of expression of YAP1-CT appears to be associated with good sensitivity and specificity for EHE variant with YAP1-TFE3 fusion and may provide additional support along with TFE3 and CAMTA1 IHC in challenging cases. This marker may also be useful in the diagnosis of RHE and CHE.
Similar content being viewed by others
Introduction
Epithelioid hemangioendothelioma (EHE) is a rare malignant vascular neoplasm that arises most commonly in the soft tissue, liver and lung of adults [1]. While it is generally less aggressive than angiosarcoma, the prognosis is variable and has been shown to depend on several factors including primary anatomic site, tumor size, and the presence of multifocal (arguably metastatic) disease [2, 3]. Histologically, it is composed of epithelioid cells with eosinophilic cytoplasm arranged in cords, nests, and as single cells, within a variably myxoid and hyalinized stroma. The tumor cells may also have intracytoplasmic vacuoles, a feature that has been likened to the early stages of angiogenesis [1].
More than 90% of EHE harbors a t(1;3)(p36;q25) translocation [4]. This cytogenetic event was identified in 2001 and subsequently shown by two independent studies in 2011 to result in WWTR1-CAMTA1 gene fusion [4,5,6]. In recent years, a distinct YAP1-TFE3 fusion has been identified as an alternative driver event in the remaining subset [7, 8]. Although EHE with YAP1-TFE3 is classified alongside EHE with WWTR1-CAMTA1 in the fifth edition World Health Organization (WHO) Classification (2020) [9], the limited data currently available suggest this variant has certain clinicopathologic differences. For example, EHE with YAP1-TFE3 fusion has been shown to affect younger patients, with a mean age of 30 years, around a decade earlier than conventional EHE [2, 7]. There is also some suggestion from a recent series that it may have a better prognosis [2]. In addition, it is morphologically distinct, typically comprising tumor cells with voluminous eosinophilic cytoplasm arranged in nests and focally forming vascular channels. This vasoformative growth pattern means that it shows histologic overlap with a much broader spectrum of endothelial neoplasms than is the case for conventional EHE, ranging from benign entities such as epithelioid hemangioma to the highly aggressive epithelioid angiosarcoma. Accurate diagnosis of this variant can therefore be particularly challenging.
In EHE with WWTR1-CAMTA1, immunohistochemistry for CAMTA1 has become established as a reliable diagnostic marker [10, 11]. For EHE with YAP1-TFE3, although TFE3 is also used in a similar manner to support the diagnosis, studies have shown it to lack specificity [8, 12]. In addition, variation in staining intensity can make interpretation of a positive result difficult. These shortcomings can therefore mean that molecular studies, such as fluorescence in situ hybridization (FISH) or next-generation sequencing (NGS), are required for confirmation.
Yes-associated protein 1 (YAP1), encoded by the partner gene in the fusion, is normally expressed in endothelial cells and functions as part of the Hippo signaling pathway. Given that the YAP1-TFE3 fusion protein in EHE typically contains only a small N-terminal region of YAP1, we hypothesized that immunohistochemistry using an antibody directed against the C-terminal region of YAP1 (YAP1-CT), may be negative in this subset (loss of expression) and potentially serve as a more specific diagnostic marker. In this study, we evaluate the role of YAP1-CT immunohistochemistry in the diagnosis of EHE with YAP1-TFE3 fusion and compare this with a range of other epithelioid vascular neoplasms that typically fall within the differential diagnosis. We also evaluated YAP1-CT in retiform hemangioendotheliomas and composite hemangioendotheliomas, which were recently shown to harbor YAP1 rearrangements in a subset of cases [13].
Materials and methods
This study was approved by the Institutional Review Board of Mass General Brigham (MGB). Cases were retrieved from the departmental and consultation files of Brigham and Women’s Hospital (BWH), including the consultation files of two of the authors (C.D.M.F. and J.L.H.). In total, 78 tumors were included in the study, encompassing 7 tumor types: EHE with YAP1-TFE3 (n = 13), EHE with WWTR1-CAMTA1 (n = 20), pseudomyogenic hemangioendothelioma (n = 10), epithelioid hemangioma (n = 19), epithelioid angiosarcoma (n = 10), retiform hemangioendothelioma (n = 4), and composite hemangioendothelioma (n = 2). The latter 6 tumors (retiform and composite hemangioendotheliomas) were recently published and known to harbor YAP1 rearrangements [13].
Representative slides of all cases were reviewed to confirm the original diagnoses. In the EHE with YAP1-TFE3 cohort, 6/13 cases were confirmed genetically. In one, the YAP1-TFE3 fusion was confirmed using a targeted next-generation sequencing platform (OncoPanel) at our institution, as previously described [14]. This demonstrated a balanced translocation generating an in-frame YAP1-TFE3 fusion connecting exon 1 of YAP1 to exon 4 of TFE3, a previously reported exon breakpoint pair. Four were included in the original series of Antonescu et al. [7] which characterized this entity. In their study, rearrangement of both YAP1 and TFE3 was confirmed in these four cases using FISH. One case was confirmed at our institution with TFE3 break-apart FISH using homebrew probes specific for the 5′ and 3′ regions of TFE3 at Xp11.23 and a probe for Xp11.1-q11.1 (DXZ1, Abbott Molecular, Des Plaines, IL). The remaining seven cases were diagnosed based on a combination of their distinctive histologic features (as outlined earlier) and immunohistochemical profile (ie. negative for CAMTA1 and positive for TFE3). Immunohistochemistry was performed on 4-μ-thick formalin-fixed paraffin-embedded tissue sections following pressure cooker antigen retrieval (Target Retrieval Solution, pH 6.1; Dako, Carpinteria, CA). The following antibody clones, dilutions, and sources were used: YAP1-CT (Clone: D8H1X; 1:100; Cell Signaling Technology, Danvers, MA), CAMTA1 (Rabbit polyclonal; 1:200; Novus Biologicals, Littleton, CO), and TFE3 (Clone: MRQ-37; 1:100; Cell Marque, Rocklin, CA). Positive control slides were stained in parallel.
Results
Clinicopathologic characteristics of epithelioid hemangioendothelioma with YAP1-TFE3 fusion
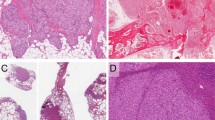
The clinicopathologic features of the EHE variant (with YAP1-TFE3 fusion) study group are summarized in Table 1 and compared with those of conventional EHE (with WWTR1-CAMTA1). In EHE variant, the median patient age was 38 years (range 14–62). Females (n = 8) slightly outnumbered males (n = 5). Of the 13 cases, 2 were multifocal. The most common location was the extremities or trunk (n = 4), followed by bone (n = 3), head and neck (n = 2), lung (n = 2), lymph nodes (n = 1), skin (n = 1), and pleura (n = 1). The latter case also involved vertebra as separate nodules, giving a total of three cases with vertebral involvement. Microscopically, EHE variants were composed of epithelioid cells with voluminous eosinophilic cytoplasm and in most cases demonstrated a focally vasoformative growth pattern (Fig. 1).
A This tumor shows frank blood vessel formation. B Immunohistochemistry for YAP1-CT shows loss of expression in tumor cells, with retained expression in pericytes and occasional non-neoplastic vessels. C This case demonstrates a solid and nested architecture. D Loss of YAP1-CT expression with a positive internal control. E This example has a sheet-like growth pattern of tumor cells with abundant eosinophilic cytoplasm and vesicular nuclei. F The tumor cells are negative for YAP1-CT while admixed stromal cells are positive.
Immunohistochemical results
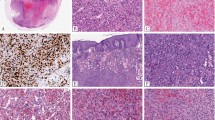
The results of YAP1-CT immunohistochemistry in EHE, retiform hemangioendothelioma, composite hemangioendothelioma, and other epithelioid vascular tumors are summarized in Table 2. Ten cases (10/13; 77%) of EHE variant showed loss of YAP1-CT expression, with no staining observed in either the nucleus or cytoplasm of tumor cells (Fig. 1). Conversely, strong nuclear YAP1-CT expression was seen in endothelial cells of normal vessels and background pericytes where present, serving as an internal positive control. Three cases showed retained YAP1-CT expression (Fig. 2): two showed weak cytoplasmic staining and one showed strong nuclear and cytoplasmic staining. Of these three cases, two were among those previously confirmed as EHE variant using molecular studies. However, the YAP1 breakpoints and YAP1 domains present in the fusion proteins of these tumors are not known, and therefore the reasons for their different staining patterns are not clear. A third case with retained YAP1-CT had not been genetically tested. Morphologically, this case had occasional foci resembling conventional EHE but by immunohistochemistry it was negative for CAMTA1 and diffusely positive for TFE3.
A This case, arising in an inguinal lymph node, was confirmed to harbor YAP1 and TFE3 rearrangements. It shows nests of tumor cells adjacent to a germinal center. B Weak retained expression of YAP1-CT was seen in the cytoplasm of tumor cells. C This example was also confirmed genetically. It comprises sheets of tumor cells with eosinophilic cytoplasm. D There was retained YAP1-CT expression with cytoplasmic and nuclear staining. E This tumor arose in the lung and had a multinodular growth pattern. F There is weak cytoplasmic positivity for YAP1-CT.
Conventional EHE cases very commonly showed retained YAP1-CT expression (19/20; 95%) (Fig. 3), with only one case having loss of expression; this CAMTA1-positive tumor showed typical histologic features of EHE. Among those with retained expression, positivity for YAP1-CT was observed in a cytoplasmic (15/20; 75%), nuclear (3/20; 15%), or both cytoplasmic and nuclear (1/20; 5%) distribution.
Loss of YAP1-CT expression was also seen in all cases of retiform hemangioendothelioma and composite hemangioendothelioma with previously confirmed YAP1 gene rearrangements (Fig. 4) [13]. All other epithelioid vascular tumors showed retained YAP1-CT expression (Fig. 5). Immunohistochemistry for TFE3 was positive in all cases of EHE variant; however, in one case the staining was weak. Equivocal staining for TFE3 was observed in the one conventional EHE tested. CAMTA1 was negative in all EHE variant cases tested (0/7) and positive in all conventional EHE (20/20).
A RHE with confirmed YAP1 rearrangement, composed of narrow branching blood vessels lined by small and uniform tumor cells with hobnail nuclei. B The tumor cells show loss of YAP1-CT expression, while background stromal cells are positive. C Composite hemangioendothelioma of the dermis, showing an admixture of epithelioid and RHE-like areas. D This case was also known to harbor YAP1 rearrangement and showed loss of YAP1-CT expression.
A Pseudomyogenic hemangioendothelioma, comprising intersecting fascicles of myoid spindle cells with eosinophilic cytoplasm. B There is retained expression of YAP1-CT. C Epithelioid hemangioma composed of blood vessels lined by epithelioid tumor cells and admixed eosinophils. D Retained cytoplasmic expression of YAP1-CT. E Epithelioid angiosarcoma, consisting of sheets of tumor cells with cytoplasmic vacuoles. F There is cytoplasmic positivity for YAP1-CT.
Discussion
In this study, we evaluated the utility of a novel antibody directed against the C-terminus of YAP1 (YAP1-CT) in the diagnosis of EHE, retiform hemangioendothelioma, and composite hemangioendothelioma, and a range of other epithelioid vascular neoplasms. We demonstrated that the EHE variant (with YAP1-TFE3 fusion) commonly shows loss of YAP1-CT expression from the nucleus and cytoplasm of tumor cells (10/13; 77%); IHC using YAP1-CT may provide additional support along with TFE3 and CAMTA1 IHC in challenging cases. Importantly, loss of YAP1-CT was seen in only 1 case of conventional EHE (CAMTA1-positive). All other vascular neoplasms showed retained YAP1-CT expression, except for retiform and composite hemangioendotheliomas (see below). We identified only three cases of EHE variant with retained YAP1-CT expression. Two of these cases had been confirmed genetically in a prior study, with FISH demonstrating rearrangement of both YAP1 and TFE3 [7]. One possible reason for the positive staining in these cases is residual YAP1 expression derived from the non-rearranged allele. Another possibility is that the fusion protein in these cases may encompass a larger region of the YAP1 transcript. The latter is difficult to verify, however, since the structure of the fusion proteins in these tumors is unknown. Genetic testing was not undertaken in the third case with positive YAP1-CT staining, although by immunohistochemistry it was negative for CAMTA1 and positive for TFE3.
EHE with YAP1-TFE3 fusion was first described in 2011 by Antonescu and colleagues [7]. Morphologically, this tumor is distinct from conventional EHE in that it demonstrates areas of bona fide blood vessel formation and more frequent solid and nested/alveolar growth patterns – the latter feature having initially prompted TFE3 immunohistochemistry in their index case. YAP1, encoded by the YAP1 gene on 11q13, is a transcriptional co-activator that shows sequence homology with WWTR1/TAZ. YAP1 and TAZ are both regulated by the Hippo signaling pathway and may be expressed in the cytoplasm or nucleus depending on their phosphorylation status. It is thought that the highly active promoter regions of YAP1 and WWTR1 lead to overexpression of TFE3 and CAMTA1 in their respective oncogenic fusions. TFE3 is located on Xp11.22 and encodes a member of the microphthalmia transcription factor family. Immunohistochemistry for TFE3 has been applied to support the diagnosis of EHE variant and is very sensitive. For example, in the series of Antonescu, all 10 cases showed strong diffuse nuclear expression [7]. However, cases with focal staining have also been described [12]. In the current study, while all cases were positive for TFE3, one showed only weak and patchy expression, requiring molecular testing (FISH) to confirm the diagnosis. The weak intensity of TFE3 staining in cases such as this make its interpretation difficult. Perhaps more problematic, however, is its imperfect specificity. In the series of Flucke et al., TFE3 positivity was reported in 19 of 22 cases of conventional EHE (with WWTR1-CAMTA1 fusion), which was occasionally more than focal [12]. Similarly, in the series of Patel and colleagues, three cases showed multifocal nuclear TFE3 staining, yet on molecular analysis harbored WWTR1-CAMTA1 fusion rather than YAP1-TFE3 [8]. A limitation of our study is that a direct comparison of TFE3 and YAP1-CT was not possible, since a subset of our cases (7/13) were diagnosed primarily based on clinical, morphologic, and immunohistochemical features (which included TFE3 positivity). Nevertheless, the high specificity of YAP1-CT that we have shown appears to complement the high sensitivity of TFE3, as determined here and in other studies, suggesting that diagnostic accuracy may be best when these biomarkers are used together.
Interestingly, YAP1 rearrangement has been characterized as a recurrent event in a growing number of other neoplasms. In a very recent study, YAP1-MAML2 fusions were identified in both retiform hemangioendothelioma and composite hemangioendothelioma, two other vascular neoplasms that behave in a locally aggressive manner [13]. We were fortunate to include six genetically confirmed cases from that series in the current study (two composite hemangioendotheliomas and four retiform hemangioendotheliomas) and found loss of YAP1-CT expression in each tumor. In addition, a recent study by Russell-Goldman and colleagues [15] demonstrated the utility of YAP1-CT in poroma and porocarcinoma, which harbor YAP1-NUTM1 or YAP1-MAML2 fusions [16]. Other tumor types which may harbor YAP1 rearrangement include sclerosing epithelioid fibrosarcoma-like sarcoma (YAP1-KMT2A) [17,18,19] and metaplastic thymoma [20], as well as rare subsets of ependymomas [21] and meningiomas [22, 23]. It will be interesting to see in future studies whether the YAP1-CT antibody is equally useful in these other contexts.
In summary, we have shown that YAP1 C-terminus immunohistochemistry is a useful diagnostic adjunct for the EHE variant with YAP1-TFE3 fusion. Overall, our results indicate that loss of YAP1-CT expression is highly specific for this tumor type and strongly supports its diagnosis. Given its moderate sensitivity (77%) however, we would recommend that this biomarker is best used alongside TFE3, which is more sensitive but less specific.
References
Weiss SW, Enzinger FM. Epithelioid hemangioendothelioma: a vascular tumor often mistaken for a carcinoma. Cancer. 1982;50:970–81.
Rosenbaum E, Jadeja B, Xu B, Zhang L, Agaram NP, Travis W, et al. Prognostic tratification of clinical and molecular epithelioid hemangioendothelioma subsets. Mod Pathol. 2020;33:591–602.
Deyrup AT, Tighiouart M, Montag AG, Weiss SW. Epithelioid hemangioendothelioma of soft tissue: a proposal for risk stratification based on 49 cases. Am J Surg Pathol. 2008;32:924–7.
Mendlick MR, Nelson M, Pickering D, Johansson SL, Seemayer TA, Neff JR, et al. Translocation t(1;3)(p36.3;q25) is a nonrandom aberration in epithelioid hemangioendothelioma. Am J Surg Pathol. 2001;25:684–7.
Tanas MR, Sboner A, Oliveira AM, Erickson-Johnson MR, Hespelt J, Hanwright PJ, et al. Identification of a disease-defining gene fusion in epithelioid hemangioendothelioma. Sci Transl Med. 2011;3:98ra82.
Errani C, Zhang L, Sung YS, Hajdu M, Singer S, Maki RG, et al. A novel WWTR1-CAMTA1 gene fusion is a consistent abnormality in epithelioid hemangioendothelioma of different anatomic sites. Genes Chromosomes Cancer. 2011;50:644–53.
Antonescu CR, Le Loarer F, Mosquera JM, Sboner A, Zhang L, Chen CL, et al. Novel YAP1-TFE3 fusion defines a distinct subset of epithelioid hemangioendothelioma. Genes Chromosomes Cancer. 2013;52:775–84.
Patel NR, Salim AA, Sayeed H, Sarabia SF, Hollingsworth F, Warren M, et al. Molecular characterization of epithelioid haemangioendotheliomas identifies novel WWTR1-CAMTA1 fusion variants. Histopathology. 2015;67:699–708.
WHO Classification of Tumours Editorial Board. WHO Classification of Tumours: Soft Tissue and Bone Tumours. 5th ed. Lyon, France: International Agency for Research on Cancer (2020).
Doyle LA, Fletcher CDM, Hornick JL. Nuclear Expression of CAMTA1 Distinguishes Epithelioid Hemangioendothelioma From Histologic Mimics. Am J Surg Pathol. 2016;40:94–102.
Shibuya R, Matsuyama A, Shiba E, Harada H, Yabuki K, Hisaoka M. CAMTA1 is a useful immunohistochemical marker for diagnosing epithelioid haemangioendothelioma. Histopathology. 2015;67:827–35.
Flucke U, Vogels RJ, de Saint Aubain Somerhausen N, Creytens DH, Riedl RG, van Gorp JM, et al. Epithelioid Hemangioendothelioma: clinicopathologic, immunohistochemical, and molecular genetic analysis of 39 cases. Diagn Pathol. 2014;9:131.
Antonescu CR, Dickson BC, Sung YS, Zhang L, Suurmeijer AJH, Stenzinger A, et al. Recurrent YAP1 and MAML2 Gene Rearrangements in Retiform and Composite Hemangioendothelioma. Am J Surg Pathol. 2020;44:1677–84.
Garcia EP, Minkovsky A, Jia Y, Ducar MD, Shivdasani P, Gong X, et al. Validation of OncoPanel: A Targeted Next-Generation Sequencing Assay for the Detection of Somatic Variants in Cancer. Arch Pathol Lab Med. 2017;141:751–8.
Russell-Goldman E, Hornick JL, Hanna J. Utility of YAP1 and NUT immunohistochemistry in the diagnosis of porocarcinoma. J Cutan Pathol. 2021;48:403–10.
Sekine S, Kiyono T, Ryo E, Ogawa R, Wakai S, Ichikawa H, et al. Recurrent YAP1-MAML2 and YAP1-NUTM1 fusions in poroma and porocarcinoma. J Clin Invest. 2019;129:3827–32.
Massoth LR, Hung YP, Nardi V, Nielsen GP, Hasserjian RP, Louissaint A Jr, et al. Pan-sarcoma genomic analysis of KMT2A rearrangements reveals distinct subtypes defined by YAP1-KMT2A-YAP1 and VIM-KMT2A fusions. Mod Pathol. 2020;33:2307–17.
Kao YC, Lee JC, Zhang L, Sung YS, Swanson D, Hsieh TH, et al. Recurrent YAP1 and KMT2A Gene Rearrangements in a Subset of MUC4-negative Sclerosing Epithelioid Fibrosarcoma. Am J Surg Pathol. 2020;44:368–77.
Puls F, Agaimy A, Flucke U, Mentzel T, Sumathi VP, Ploegmakers M, et al. Recurrent Fusions Between YAP1 and KMT2A in Morphologically Distinct Neoplasms Within the Spectrum of Low-grade Fibromyxoid Sarcoma and Sclerosing Epithelioid Fibrosarcoma. Am J Surg Pathol. 2020;44:594–606.
Vivero M, Davineni P, Nardi V, Chan JKC, Sholl LM. Metaplastic thymoma: a distinctive thymic neoplasm characterized by YAP1-MAML2 gene fusions. Mod Pathol. 2020;33:560–5.
Pajtler KW, Witt H, Sill M, Jones DT, Hovestadt V, Kratochwil F, et al. Molecular Classification of Ependymal Tumors across All CNS Compartments, Histopathological Grades, and Age Groups. Cancer Cell. 2015;27:728–43.
Schieffer KM, Agarwal V, LaHaye S, Miller KE, Koboldt DC, Lichtenberg T, et al. YAP1-FAM118B Fusion Defines a Rare Subset of Childhood and Young Adulthood Meningiomas. Am J Surg Pathol. 2021;45:329–40.
Sievers P, Chiang J, Schrimpf D, Stichel D, Paramasivam N, Sill M, et al. YAP1-fusions in pediatric NF2-wildtype meningioma. Acta Neuropathol. 2020;139:215–8.
Acknowledgements
The authors thank Ms. Mei Zheng (Brigham and Women’s Hospital, Boston, MA) for assistance in performing immunohistochemistry.
Funding
Departmental funds were used to support the costs of immunohistochemistry.
Author information
Authors and Affiliations
Contributions
J.L.H. designed the study; C.D.M.F. and J.L.H. provided materials and reagents; W.J.A. acquired and analyzed the data; W.J.A. wrote the paper; all authors read and approved the final paper.
Corresponding author
Ethics declarations
competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by the Institutional Review Board of the Brigham and Women’s Hospital.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Anderson, W.J., Fletcher, C.D.M. & Hornick, J.L. Loss of expression of YAP1 C-terminus as an ancillary marker for epithelioid hemangioendothelioma variant with YAP1-TFE3 fusion and other YAP1-related vascular neoplasms. Mod Pathol 34, 2036–2042 (2021). https://doi.org/10.1038/s41379-021-00854-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-021-00854-2