Abstract
Pulmonary surfactant is essential to maintain alveolar patency, and invariably fatal neonatal lung disease has been recognized to involve mutations in the genes encoding surfactant protein-B or ATP-binding cassette transporter family member ABCA3. The lipid transporter ABCA3 targets surfactant phospholipids to lamellar bodies that are lysosomal-derived organelles of alveolar type II cells. ABCA3−/− mice have grossly reduced surfactant phosphatidyl glycerol levels and die of respiratory failure soon after birth. We studied lung biopsy samples of two siblings with a novel homozygous ABCA3 mutation at nucleotide position 578 (c.578C>G), leading to a Pro193Arg amino-acid exchange, who died at 55 and 105 days of age. Light microscopy revealed thickened alveolar septa with abundant myxoid interstitial matrix, marked hyperplasia of type II pneumocytes, desquamation of alveolar macrophages and focal alveolar proteinosis. Surfactant protein-B was detected by immunohistochemistry after antigen retrieval. Transmission electron microscopy showed rare cytoplasmic inclusions with concentric membranes and eccentrically placed electron-dense aggregates. These ‘fried-egg’-appearing lamellar bodies differed both from normal lamellar bodies and the larger, poorly formed composite bodies with multiple vesicular inclusions observed in surfactant protein-B deficiency. In conclusion, our findings underscore that the implications of interstitial lung disease in infant lungs differ from those in adults. In infants with a desquamative interstitial pneumonitis pattern, surfactant or ABCA3 mutations should be evaluated. Importantly, these findings support the notion that electron microscopy is useful in distinguishing between surfactant protein-B and ABCA3 deficiency, and has an important role in evaluating biopsies or autopsies of term infants with unexplained severe respiratory failure and interstitial lung disease.
Similar content being viewed by others
Main
Pulmonary surfactant is a highly surface-active, complex mixture of lipids and proteins that lowers surface tension and prevents atelectasis.1 Surfactant is synthesized in alveolar type II cells, stored in lamellar bodies and secreted into the alveolar space by exocytosis. The most abundant lipid component is phosphatidylcholine, and the major surfactant proteins are surfactant protein-A, -B, -C and -D.2
In preterm infants, insufficient amounts of surfactant produced by type II cells lead to respiratory distress syndrome.3 In term infants, inherited mutations in the genes coding for surfactant protein-B, SFTPB (surfactant protein-B deficiency) and SFTPC have been identified as cause of respiratory failure.4, 5, 6 Histopathology of surfactant protein-B deficiency resembles the adult form of alveolar proteinosis or a desquamative interstitial pneumonitis (DIP)-like pattern.4, 7
In addition to SFTPB and SFTPC mutations, inherited severe neonatal respiratory distress has recently been attributed to mutations in the ATP-binding cassette A3 transporter (ABCA3) gene.1, 8, 9, 10, 11, 12 ABCA3 is a member of the ATP-binding cassette transporter family of proteins that actively transport a wide range of substrates across cell and intracellular membranes.11, 13 In infants with severe neonatal respiratory distress due to ABCA3 mutations, the histological features correspond to a pattern of non-specific interstitial pneumonia.8 Such mutations in the ABCA3 transporter gene are increasingly being recognized.
We studied members of a consanguinous family, two of which died from congenital respiratory distress syndrome, by conventional histology, ultrastructure as well as by ABCA3 gene sequencing to look for a responsible ABCA3 mutation and to determine phenotypic–genotypic correlation.
Materials and methods
Patients
Two of four infants of an inbred Algerian family suffered from progressive respiratory distress with onset in the first hours after birth. The girl, born in 2000, and the boy, born in 2006, were admitted to the neonatal intensive care unit for cyanosis and tachydyspnea on the first day of life and treated with increasing concentrations of oxygen. Chest X-rays showed diffuse opacities. Mechanical ventilation was started at day 3 or 4 of life, respectively. The patients did not respond to exogenous surfactant administration, inhaled nitric oxide, intravenous prostacyclin, steroids, chloroquine or immunoglobulins. Surfactant protein-B was detected in bronchoalveolar lavage fluid. Open lung biopsies were performed at the age of 2 and 7 weeks, respectively. The infants died of relentless hypoxemic respiratory failure at 105 and 55 days of life. Two siblings, parents and grandparents were asymptomatic. Informed consent was given for mutational analysis.
Conventional Histology
Lung tissue was formalin-fixed and paraffin-embedded for conventional histologic stains.
Immunohistochemistry
Formalin-fixed and paraffin-embedded lung tissue was submitted for immunohistochemical investigation. Immunohistochemistry for surfactant protein-A, surfactant precursor protein-B and mature surfactant protein-B was performed according to standard protocols (surfactant protein-A mouse monoclonal 1:200 and surfactant precursor protein-B mouse monoclonal 1:50, Novocastra, Newcastle upon Tyne, UK; and surfactant protein-B mouse monoclonal 1:25, Lab Vision, Fremont, CA, USA). Antigen retrieval was achieved by microwave at 98°C for 30 min in citrate buffer.
Ultrastructural Examination
Small pieces of lung tissue (<1 mm) were glutaraldehyde fixed, epon resin embedded, semithin and ultrathin cut, uranyl acetate and lead citrate stained for ultrastructural investigation at an FEI Morgagni transmission electron microscope with digital camera equipment.
ABCA3 Gene Mutation Analysis
Genomic DNA was extracted from EDTA whole blood using the DNA Midi kit (Qiagen, Hilden, Germany), and from fresh-frozen lung tissue and deparaffinized-paraffin sections using the QIAamp DNA Mini kit (Qiagen), as described in the manufacturer's protocol. The 30 coding exons of the ABCA3 gene of the index patient and individual exons from five family members were PCR-amplified with flanking, intronic sequences and the exon sequences, and splice sites were compared to the genomic ABCA3 wild-type sequence as derived using the NM_001089.1 mRNA as described in Brasch et al.10 PCR products were sequenced by direct cycle sequencing using an ABI Prism 3130xl Genetic Analyser capillary sequencer and BigDye terminator (Applied Biosystems, Foster City, CA, USA). The primer sequences, annealing temperatures and length of PCR products are provided in the Supplementary Table.
Results
Conventional Histology
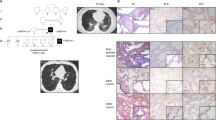
Lung histology demonstrated chronic pneumonitis of infancy with a combination of morphologic features of non-specific interstitial pneumonia, pulmonary alveolar proteinosis and a DIP pattern (Figure 1). Features of non-specific interstitial pneumonia consisted of thickened alveolar septa with increase in cell content, abundant myxoid interstitial matrix and minor loose fibrosis. Resulting slit-like airspaces indicated severe acinar dysplasia. Smooth muscle hyperplasia in the bronchiolar–alveolar duct area was present as often seen in ‘stiff’ lungs with interstitial pneumonitis (Figure 2). There was marked hyperplasia of type II pneumocytes. Focally, an alveolar accumulation of finely granular, eosinophilic and PAS-positive material was found (Figure 2), as seen in pulmonary alveolar proteinosis. Similarly, desquamation of alveolar macrophages was observed, typical of a DIP pattern.
Conventional histology (2 weeks, patient boy). (a) Low power of open lung biopsy shows interstitial fibroplasia (Alcian blue PAS × 50). (b) Interalveolar septa are widened and occupied by loose connective tissue. Alveoli are collapsed (Alcian blue PAS × 200). (c) Lung lobule with massive pneumocyte type II hyperplasia (Alcian blue PAS × 400). (d) Intraalveolar deposition of PAS-positive material (Alcian blue PAS × 400). (e) Intraalveolar deposits of finely granular material (Alcian blue PAS × 630). (f) Massive pneumocyte type II hypertrophy, hyperplasia and desquamation (Alcian blue PAS × 630).
Conventional histology (6 weeks, patient girl) and immunohistochemistry. (a) Low power of lung biopsy at 6 weeks shows smooth muscle hyperplasia in the bronchiolar–alveolar duct area (PAS × 100). (b) Persisting pneumocyte type II hyperplasia, intraluminal deposition of finely granular PAS-positive material and desquamation (PAS × 400). (c) Immunohistochemistry for surfactant precursor protein-B shows fine granular cytoplasmic expression pattern in alveolar pneumocytes type II ( × 400). (d) Immunohistochemistry for surfactant protein-A demonstrates diffuse expression in alveolar pneumocytes and intense staining of alveolar exudate ( × 400).
Immunohistochemistry
Immunohistochemistry for surfactant precursor protein-B and mature surfactant protein-B showed granular expression in type II pneumocytes. Immunohistochemistry for surfactant protein-A demonstrated homogeneous cytoplasmic expression. The intra-alveolar accumulated material stained for surfactant protein-A and focally for surfactant precursor protein-B (Figure 2).
Ultrastructure
Ultrastructurally, hyperplastic type II pneumocytes contained rare cytoplasmic inclusions with tightly packed concentric membranes and an aggregate of electron-dense material within the cytoplasmic inclusions (‘fried-egg’ appearance) as described by Cutz et al1 and Edwards et al.14 Regular lamellar bodies as in normal type II pneumocytes or composite bodies typical of surfactant protein-B deficiency were absent (Figures 3 and 4).
Electron microscopy (patient boy). (a) Apical pneumocyte type II cytoplasm is occupied with small lamellar bodies of compact texture and irregular, focally eccentric electron dense deposits ( × 22 000). (b) Lamellar body with irregular electron dense deposits ( × 36 000). (c) Lamellar body composed of compact concentric membranes with vesicular core ( × 32 000). (d) Even more compact lamellar body architecture of smaller size ( × 56 000). (E) Small compact lamellar bodies with eccentric electron dense deposits ( × 36 000). (f) Small abnormally compact lamellar bodies contain a vesicular core ( × 58 000).
ABCA3 Gene Mutation Analysis
Mutation analysis was performed on seven individuals. All 30 coding exons of the ABCA3 gene were submitted to sequence analysis in the second patient. The patient was found to be homozygous for a point mutation of base pair 578 C>G in exon 7 (c.578C>G, numbering beginning at the ATG start as given in mRNA NM_001089.1), resulting in amino-acid exchange Pro193Arg (Figure 5). DNA extracted from paraffin-embedded material from the first patient revealed homozygosity for the same mutation. Both asymptomatic parents, an asymptomatic sister and the asymptomatic grandmother on the mother's side were heterozygous for this mutation. A healthy brother showed an ABCA3 wild-type sequence. The mutation was not found in 54 controls.
Structural model of ABCA3 protein and location of novel ABCA3 mutation. ABCA3 is constituted by 12 putative membrane-spanning helices, two extracellular domains (ECD) and two nucleotide-binding domains (NBD). A and B indicates critical conserved motifs Walker A and B, respectively, in the two nucleotide-binding domains. The red circle indicates the novel mutation Pro193Arg found in this study, located in the first extracellular domain.
Discussion
Pulmonary homeostasis at birth and later in life depends on the precise regulation of synthesis and secretion of surfactant proteins-B and -C, as well as the formation of lamellar bodies. Surfactant proteins-B and -C are synthesized and packaged with surfactant phospholipids in lamellar bodies. Normal lamellar body formation requires surfactant protein-B and ABCA3 as a member of the ABC family of ATP-dependent membrane-associated transport proteins. Mutations in SFTPB, SFTPC and ABCA3 genes disrupt type II alveolar pneumocyte function and cause abnormalities in surfactant homeostasis.15, 16 ABCA3 gene mutations have recently been described in fatal respiratory disease in newborns and severe chronic lung disease in infancy.1, 8, 10 Expression of surfactant proteins-B, -C and ABCA3 are coregulated during late gestation by thyroid transcription factor 1 and forkhead box a2. SFTP-B and -C mutations account for a relatively small percentage of severe respiratory failure in newborns.15 Clinical manifestation has been described as similar between SFTP-B, -C and ABCA3 mutations.15 Conventional histology shows similar overlapping features.
We have studied lung conventional morphology, immunohistochemistry and ultrastructure in two patients of a consanguinous family and correlated morphology with ABCA3 gene analysis in affected and non-affected family members, as well as 54 control patients.
The histopathologic findings in the lung biopsies of our patients revealed a variable combination of non-specific interstitial pneumonia, a DIP-like pattern and pulmonary alveolar proteinosis-like features as are characteristic of the so-called ‘chronic pneumonitis of infancy’.10 This term was originally coined by Katzenstein et al17 and denotes alveolar septal thickening, striking type II pneumocyte hyperplasia and a granular alveolar exudate with numerous macrophages.19 While alveolar septal thickening due to fibroblast proliferation, type II pneumocyte hyperplasia and focal intraalveolar accumulation of fine granular, eosinophilic material in conjunction with alveolar macrophages as the typical conventional histologic elements of this chronic pneumonitis of infancy have been described in association with ABCA3 mutations,8, 10, 11 there is considerable heterogeneity in the findings between different infants. It has been emphasized that the light microscopy findings are not specific for ABCA3 gene mutations,9 as the morphology is similar to those observed in infants with SFTPB and SFTPC gene mutations. Rather, such morphologic findings are suggestive of an inborn error-disrupting surfactant metabolism, but are not specific for the disorder.9 Morphologic findings of congenital chronic pneumonitis of infancy should trigger immunohistochemical and ultrastructural investigation to search for abnormal surfactant metabolism and surfactant lamellar body structure.
The importance of ultrastructural investigation in the diagnosis of congenital surfactant deficiencies has recently been outlined in the recommendations of the Children's Interstitial Lung Disease Pathology Co-operative Group (chILD Pathology Co-operative Group) in the protocol for the handling of tissue obtained by operative lung biopsy.20 Besides adequate preservation of tissue for microbiological cultures and molecular studies, in infants and young children, collection of tissue in glutaraldehyde for electron microscopy is strongly recommended for lung biopsies. Our study emphasizes the usefulness of setting aside a small piece of lung tissue for ultrastructural investigation in neonatal patients with clinical presentation of interstitial lung disease. An electron microscopy study should be part of the evaluation of an infant with unexplained severe respiratory failure who undergoes a lung biopsy, or in the autopsy of a full-term infant who dies from hypoxic respiratory failure of unknown cause.20
In patients with ABCA3 gene mutations, abnormal processing of surfactant protein-B has been reported, resulting in decreased expression of mature surfactant protein-B in alveolar pneumocytes.10 Decreased surfactant protein-B expression with granular appearance was found in immunohistochemistry without antigen retrieval and recovered with antigen retrieval. In our patient, we found a focally prominent granular pattern of pneumocyte surfactant protein-B expression in line with previous findings.
Electron microscopic investigation is an important tool in the investigation of congenital surfactant deficiency. In normal type II pneumocytes, surfactant-containing mature lamellar bodies occupy the apical cytoplasm and appear as onion-skin arrangement of regular concentric lamellae on ultrastructural examination.18 In contrast, in congenital surfactant deficiency, ultrastructural investigation shows a characteristically abnormal lamellar body pattern. Importantly, the ultrastructural aspect is distinct for each type of congenital surfactant deficiency and may be applied for morphologic discrimination (Table 1). Mature lamellar bodies are absent in type II pneumocytes in surfactant protein-B deficiency, which features composite bodies with membranous and vesicular structures instead.14, 21 Similarly, deficiency of mature lamellar bodies in alveolar type II cells has been described in ABCA3 mutations.1, 14 In contrast to surfactant protein-B deficiency, in ABCA3 mutations, a different, characteristic phenotype of lamellar bodies has been described.1, 14 Cytoplasmic inclusions with tightly packed concentric membranes and distinctive electron dense aggregates of ‘fried-egg’ appearance are present in congenital surfactant deficiency caused by ABCA3 mutations.14 These inclusions may now be regarded as characteristic of ABCA3 mutations, and should trigger molecular ABCA3 mutation analysis in the patient. In the rarer SFTPC mutations, lamellar bodies are characterized by vesicular lamellar bodies with dense cores.22, 23
Pediatric interstitial lung diseases are a heterogeneous group of disorders, and are gradually being better understood. The types of interstitial lung disease in adults differ from those seen in children, and the course and prognoses differ between children and adults.11, 24, 25 Consequently, it is important to recognize that a DIP pattern in children differs from and has entirely different implications from that found in adults. Both in children and adults, the major histopathologic feature of a DIP pattern is the presence of alveolar macrophage accumulations.26 In addition, in pediatric patients, hyperplasia of type II pneumocytes is described as characteristic.27 In contrast to a DIP pattern as seen in DIP respiratory bronchiolitis-associated interstitial lung disease in adults, a DIP pattern in children is not linked to smoking.26, 19 Whereas a DIP pattern in adults is associated with a good prognosis and 10-year survival of 100%,28 congenital manifestation is associated with a high mortality rate. Over recent years, surfactant deficiencies as causative factors have been delineated in a minority of patients, particularly with congenital manifestation (Table 1).11, 16 As a consequence, in infants with a DIP-like pattern, mutations in surfactant or ABCA3 genes should be sought.
Morphologic findings on conventional histology are considerably heterogeneous between different infants but rather similar for infants with SFTPB, SFTPC and ABCA3 gene mutations. Therefore, conventional histology is suggestive for an inborn error disrupting surfactant metabolism, but has not been regarded as specific for a particular surfactant disorder.11 The age of onset as currently known is typically earlier for SFTPB and ABCA3 mutations, and has been found more variable in patients with SFTPC mutations. The clinical course is characteristically rapid for SFTPB and ABCA3 mutations and typically more protracted for patients with SFTPC mutations (Table 1). However, recently, in a number of older patients with a milder course, ABCA3 mutations have been documented.9 Therefore, it may be speculated that ABCA3 mutations, if sought in a population-based study, may prove to be far more widespread than currently recognized.11
ABCA3 mutations are inherited in an autosomal recessive manner and involve the gene encoding the ABC transporter A3.8 ABC transporters are a superfamily of highly conserved membrane proteins that transport a wide variety of substrates across cell membranes.13 The ABCA3 gene is located on chromosome 16p13.3 and encodes a 1704 amino-acid protein highly expressed in the lung. It has been localized to the limiting membrane of lamellar bodies.29, 30, 31 The ABCA3 transporter is thought to be involved in the traffic of specific lipids essential for the formation of mature lamellar bodies.32 ABCA3 mutants in fatal surfactant deficiency have been characterized and classified.2 The role of ABCA3 mutations depends on the codon affected. The mechanism of surfactant deficiency can be classified into two categories: abnormal intracellular trafficking (type I) and decreased ATP hydrolysis activity with normal intracellular trafficking (type II).2 Homozygous type II mutations have not been reported. Patients with type I/type II compound heterozygous ABCA3 mutations died of surfactant deficiency during the neonatal period, and lamellar bodies from a patient with a type I/type II heterozygous mutation were reported to be smaller than those from normal lung tissue.8 However, type II ABCA3 mutations on one allele do not result in fatal surfactant deficiency and suggest that a sufficient level of ATP hydrolysis activity of the ABCA3 protein encoded in the other allele is essential for the function of the ABCA3 protein, maturation of the lamellar bodies and surfactant metabolism.2 Therefore, intracellular trafficking and/or ATP hydrolysis activity is dramatically impaired in the ABCA3 mutant proteins so far found in fatal surfactant deficiency. Such severe ABCA3 protein dysfunction is implicated in the severe phenotype of these patients.
ABCA3 mutations have also been implied in pediatric interstitial lung disease with a milder phenotype than that of fatal surfactant deficiency.9 In patients between 1 month and older than 16 years with pediatric interstitial lung disease, ABCA3 mutations in codon 292 have been found to cause a milder phenotype with survival into young adulthood.9 The finding of patients with disease who have an ABCA3 mutation identified on only one allele suggests that there are ABCA3 mutations, some of which could be associated with an even milder phenotype.9
The specific ABCA3 gene mutation found in our patients is the point mutation 578C>G located in exon 7 and replaces the amino-acid prolin with arginin in codon 193 of the first extracellular domain (Figure 5). This ABCA3 mutation has not been described previously.8, 10 Proline is known to have a strong structural effect due to its long side chain and is therefore also referred to as ‘helix breaker’.33 As a consequence, the amino-acid exchange Pro193Arg implies an alteration of secondary molecule structure and is predicted to result in massive functional impairment of ABCA3. It remains to be determined if intracellular trafficking, nucleotide-binding affinity or ATP hydrolysis activity is more severely affected in this particular type of ABCA3 gene mutation. The conventional and ultrastructural morphologic findings in our patients correlate with a severe phenotype associated with homozygous Pro193Arg ABCA3 gene mutation. Absence of respiratory symptoms in the three Pro193Arg heterozygous adult patients implies sufficiently retained ABCA3 protein function in the heterozygous state. The c.578C>G ABCA3 point mutation was not found in 54 control patients (108 alleles), rendering a DNA polymorphism unlikely.
In summary, we report a previously undescribed mutation of the ABCA3 gene with predicted impact on ABCA3 protein structure and function. Histopathologic and ultrastructural electron microscopic investigations yield characteristic morphologic findings and play a key role in the triage for genetic sequencing in infants with neonatal lung disease and congenital surfactant deficiency. Unlike in adults, in infants with a DIP-like pattern, surfactant or ABCA3 gene mutations should be evaluated. Ultrastructural evidence for ‘fried-egg’-like inclusions should trigger ABCA3 sequencing.
References
Cutz E, Wert SE, Nogee L, et al. Deficiency of lamellar bodies in alveolar type II cells associated with fatal respiratory disease in a full-term infant. Am J Respir Crit Care Med 2000;161:608–614.
Matsumura Y, Ban N, Ueda K, et al. Characterization and classification of ATP-binding cassette transporter ABCA3 mutants in fatal surfactant deficiency. J Biol Chem 2006;281:34503–34514.
Avery ME, Mead J . Surface properties in relation to atelectasis and hyaline membrane disease. Am J Dis Child 1959;97:517–523.
Nogee LM, de Mello DE, Dehner LP, et al. Deficiency of pulmonary surfactant protein B in congenital alveolar proteinosis. N Engl J Med 1993;328:406–410.
Lawson WE, Grant SW, Ambrosini V, et al. Genetic mutations in surfactant protein C are a rare cause of sporadic cases of IPF. Thorax 2004;59:977–980.
Tredano M, Griese M, Brasch F, et al. Mutation of SFTPC in infantile pulmonary alveolar proteinosis with or without fibrosing lung disease. Am J Med Genet A 2004;126:18–26.
de la Fuente AA, Voorhout WF, de Mello DE . Congenital alveolar proteinosis in the Netherlands: a report of five cases with immunohistochemical and genetic studies of surfactant apoproteins. Ped Pathol Lab Med 1997;17:221–231.
Shulenin S, Nogee LM, Annilo T, et al. ABCA3 gene mutations in newborns with fatal surfactant deficiency. N Engl J Med 2004;350:1296–1303.
Bullard JE, Wert SE, Whitsett JA, et al. ABCA3 mutations associated with pediatric interstitial lung disease. Am J Respirat Crit Care Med 2005;172:1026–1031.
Brasch F, Schirmanski S, Mühlfeld C, et al. Alteration of the pulmonary surfactant system in full-term infants with hereditary ABCA3 deficiency. Am J Respir Crit Care Med 2006;174:571–580.
Bullard JE, Wert SE, Nogee LM . ABCA3 deficiency: neonatal respiratory failure and interstitial lung disease. Sem Perinatol 2006;30:327–334.
Prestridge A, Wooldridge J, Deutsch G, et al. Persistent tachypnea and hypoxia in a 3-month-old term infant. J Pediatr 2006;149:702–706.
Cheong N, Madesh M, Gonzales LW, et al. Functional and trafficking defects in ATP binding cassette A3 mutants associated with respiratory distress syndrome. J Biol Chem 2006;281:9791–9800.
Edwards V, Cutz E, Viero S . Ultrastructure of lamellar bodies in congenital surfactant deficiency. Ultrastruct Pathol 2005;29:503–509.
Whitsett JA, Wert SE, Xu Y . Genetic disorders of surfactant homeostasis. Biol Neonate 2005;87:283–287.
Nogee LM . Genetic mechanisms of surfactant deficiency. Biol Neonate 2004;85:314–318.
Katzenstein AL, Gordon LP, Oliphant M, et al. Chronic pneumonitis of infancy: a unique form of interstitial lung disease occurring in early childhood. Am J Surg Pathol 1995;19:439–447.
Nicholson AG, Kim H, Corrin B, et al. The value of classifying interstitial pneumonitis in childhood according to defined histological patterns. Histopathology 1998;33:203–211.
Fan LL, Deterding RR, Langston C . Pediatric interstitial lung disease revisited. Pediatr Pulmonol 2004;38:369–378.
Langston C, Patterson K, Dishop MK, chILD Pathology Co-operative Group, et al. A protocol for the handling of tissue obtained by operative lung biopsy: recommendations of the chILD pathology co-operative group. Pediatr Dev Pathol 2006;9:173–180.
DeMello DE, Heyman S, Phelps DS, et al. Ultrastructure of lung in surfactant protein B deficiency. Am J Respir Cell Mol Biol 1994;11:230–239.
Brasch F, Mueller KM . Classification of pulmonary alveolar proteinosis in newborns, infants and children. Pathologe 2004;25:299–310.
Brasch F, Griese M, Tredano M, et al. Interstitial lung disease in a baby with a de novo mutation in the SFTPC gene. Eur Respir J 2004;24:30–39.
Fan LL, Langston C . Pediatric interstitial lung disease: children are not small adults. Am J Respir Crit Care Med 2002;165:1466–1467.
Fan LL, Deterding RR, Langston C . Pediatric interstitial lung disease revisited. Pediatr Pulmonol 2004;38:369–378.
Elkin SL, Nicholson AG, duBois RM . Desquamative interstitial pneumonia and respiratory bronchiolitis-associated interstitial lung disease. Semin Respir Crit Care Med 2001;22:387–397.
Travis WD, Matsui K, Moss J, et al. Idiopathic nonspecific interstitial pneumonia: prognostic significance of cellular and fibrosing patterns: survival comparison with usual interstitial pneumonia and desquamative interstitial pneumonia. Am J Surg Pathol 2000;24:19–33.
Fan LL, Langston C . Chronic interstitial lung disease in children. Pediatr Pulmonol 1993;16:184–196.
Weaver TE, Na CL, Stahlmann M . Biogenesis of lamellar bodies, lysosome-related organelles involved in storage and secretion of pulmonary surfactant. Semin Cell Dev Biol 2002;13:263–270.
Yamano G, Funahashi H, Kawanami O, et al. ABCA3 is a lamellar body membrane protein in human lung alveolar type II cells. FEBS Lett 2001;508:221–225.
Mulugeta S, Gray JM, Notarfrancesco KL, et al. Identification of LBM180, a lamellar body limiting membrane protein of alveolar type II cells, as the ABC transporter protein ABCA3. J Biol Chem 2002;277:22147–22155.
Nagata K, Yamamoto A, Ban N, et al. Human ABCA3, a product of a responsible gene for abca3 for fatal surfactant deficiency in newborns, exhibits unique ATP hydrolysis activity and generates intracellular multilamellar vesicles. Biochem Biophys Res Commun 2004;324:262–268.
Prevelige Jr PE, Fasman GD . Chou-Fasman prediction of the secondary structure of proteins: the Chou–Fasman–Prevelige algorithm. In: Fasman G (ed). Prediction of Protein Structure and the Principles of Protein Conformation. Plenum Publishing Corp: New York, 1989, pp 391–416.
Acknowledgements
We are indebted to Ms Claudia Lautenschlager for skillful performance of the ultrastructural investigations, Mss Rita Epper and Susanne Grieshaber for immunohistochemistry.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure/conflict of interest
The authors have no conflict of interest to disclose.
Supplementary Information accompanies the paper on Modern Pathology website (http://www.nature.com/modpathol)
Supplementary information
Rights and permissions
About this article
Cite this article
Bruder, E., Hofmeister, J., Aslanidis, C. et al. Ultrastructural and molecular analysis in fatal neonatal interstitial pneumonia caused by a novel ABCA3 mutation. Mod Pathol 20, 1009–1018 (2007). https://doi.org/10.1038/modpathol.3800928
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800928
Keywords
This article is cited by
-
Surfactant protein disorders in childhood interstitial lung disease
European Journal of Pediatrics (2021)
-
The biology of the ABCA3 lipid transporter in lung health and disease
Cell and Tissue Research (2017)
-
Interstitielle Prozesse der Lunge im Kindesalter
Der Pathologe (2017)
-
A large kindred of pulmonary fibrosis associated with a novel ABCA3 gene variant
Respiratory Research (2014)
-
Effect of subchronic exposure to arsenic on levels of essential trace elements in mice brain and its gender difference
BioMetals (2013)