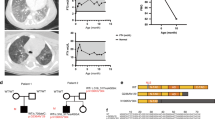
Abstract
Biallelic pathogenic variants in the surfactant protein (SP)-B gene (SFTPB) have been associated with fatal forms of interstitial lung diseases (ILD) in newborns and exceptional survival in young children. We herein report the cases of two related adults with pulmonary fibrosis due to a new homozygous SFTPB pathogenic variant, c.582G>A p.(Gln194=). In vitro transcript studies showed that this SFTPB synonymous pathogenic variant induces aberrant splicing leading to three abnormal transcripts with the preservation of the expression of a small proportion of normal SFTPB transcripts. Immunostainings on lung biopsies of the proband showed an almost complete loss of SP-B expression. This hypomorphic splice variant has thus probably allowed the patients’ survival to adulthood while inducing an epithelial cell dysfunction leading to ILD. Altogether, this report shows that SFTPB pathogenic variants should be considered in atypical presentations and/or early-onset forms of ILD particularly when a family history is identified.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Nogee LM, de Mello DE, Dehner LP, Colten HR. Brief report: deficiency of pulmonary surfactant protein B in congenital alveolar proteinosis. N Engl J Med. 1993;328:406–10.
Nogee LM, Garnier G, Dietz HC, Singer L, Murphy AM, deMello DE, et al. A mutation in the surfactant protein B gene responsible for fatal neonatal respiratory disease in multiple kindreds. J Clin Invest. 1994;93:1860–3.
Nathan N, Borensztajn K, Clement A. Genetic causes and clinical management of pediatric interstitial lung diseases. Curr Opin Pulm Med. 2018;24:253–9.
Hamvas A. Inherited surfactant protein-B deficiency and surfactant protein-C associated disease: clinical features and evaluation. Semin Perinatol. 2006;30:316–26.
Nathan N, Taam RA, Epaud R, Delacourt C, Deschildre A, Reix P, et al. A national internet-linked based database for pediatric interstitial lung diseases: the French network. Orphanet J Rare Dis. 2012;7:40.
Kurath-Koller S, Resch B, Kraschl R, Windpassinger C, Eber E. Surfactant protein B deficiency caused by homozygous C248X mutation-a case report and review of the literature. AJP Rep. 2015;5:e53–9.
Turcu S, Ashton E, Jenkins L, Gupta A, Mok Q. Genetic testing in children with surfactant dysfunction. Arch Dis Child. 2013;98:490–5.
Ueno T, Linder S, Na C-L, Rice WR, Johansson J, Weaver TE. Processing of pulmonary surfactant protein B by napsin and cathepsin H. J Biol Chem. 2004;279:16178–84.
Brasch F, Ochs M, Kahne T, Guttentag S, Schauer-Vukasinovic V, Derrick M, et al. Involvement of napsin A in the C- and N-terminal processing of surfactant protein B in type-II pneumocytes of the human lung. J Biol Chem. 2003;278:49006–14.
Palomar LM, Nogee LM, Sweet SC, Huddleston CB, Cole FS, Hamvas A. Long-term outcomes after infant lung transplantation for surfactant protein B deficiency related to other causes of respiratory failure. J Pediatr. 2006;149:548–53.
Eldridge WB, Zhang Q, Faro A, Sweet SC, Eghtesady P, Hamvas A, et al. Outcomes of lung transplantation for infants and children with genetic disorders of surfactant metabolism. J Pediatr. 2017;184:157–64.e2.
Ballard PL, Nogee LM, Beers MF, Ballard RA, Planer BC, Polk L, et al. Partial deficiency of surfactant protein B in an infant with chronic lung disease. Pediatrics. 1995;96:1046–52.
López-Andreu JA, Hidalgo-Santos AD, Fuentes-Castelló MA, Mancheño-Franch N, Cerón-Pérez JA, Esteban-Ricós MJ, et al. Delayed presentation and prolonged survival of a child with surfactant protein B deficiency. J Pediatr. 2017;190:268–70.e1.
Dunbar AE 3rd, Wert SE, Ikegami M, Whitsett JA, Hamvas A, White FV, et al. Prolonged survival in hereditary surfactant protein B (SP-B) deficiency associated with a novel splicing mutation. Pediatr Res. 2000;48:275–82.
Hamouda S, Trabelsi I, de Becdelièvre A, Boussetta K. Difficulties in the treatment of an infant survivor with inherited surfactant protein-B deficiency in Tunisia. Ann Thorac Med. 2022;17:132–5.
Nesslein LL, Melton KR, Ikegami M, Na C-L, Wert SE, Rice WR, et al. Partial SP-B deficiency perturbs lung function and causes air space abnormalities. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1154–61.
Katzen J, Beers MF. Contributions of alveolar epithelial cell quality control to pulmonary fibrosis. J Clin Invest. 2020;130:5088–99.
Wang JY, Young LR. Insights into the pathogenesis of pulmonary fibrosis from genetic diseases. Am J Respir Cell Mol Biol. 2022;67:20–35.
Garcia CK. Idiopathic pulmonary fibrosis: update on genetic discoveries. Proc Am Thorac Soc. 2011;8:158–62.
van Moorsel CHM, van der Vis JJ, Grutters JC. Genetic disorders of the surfactant system: focus on adult disease. Eur Respir Rev. 2021;30:200085.
Sutton RM, Bittar HT, Sullivan DI, Silva AG, Bahudhanapati H, Parikh AH, et al. Rare surfactant-related variants in familial and sporadic pulmonary fibrosis. Hum Mutat. 2022;43:2091–101.
Acknowledgements
We thank the patients and their family. We thank the French national networks for rare lung diseases: Centre de référence des maladies respiratoires rares (RespiRare), Centre de référence des maladies pulmonaires rares (OrphaLung) and Filière de soins pour les maladies respiratoires rares (RespiFIL). The ILD cohort has been developed in collaboration with the Rare Disease Cohort (RaDiCo)-ILD project (ANR-10-COHO-0003), the ERS Clinical research collaboration for chILD-EU and the COST Innovative Grant OpenILD CIG16125.
Funding
Our work is supported by the Legs Poix from the Chancellerie des Universités (grants 2013 n°1305, 2014 n°1405, 2015 n°1015, 2016 n°2077, 2017 n°DP2017/1860 and 2022 n°2022000594).
Author information
Authors and Affiliations
Contributions
TD and NN performed the functional studies and wrote the manuscript that was reviewed by all the authors. ML contributed to the data analysis. PD provided his expertise in functional studies. GP and ALB were in charge of the patients. VN and FDL performed the molecular diagnosis under the supervision of ML and SA. AC performed the histological analyses.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Written informed consents were obtained from the two patients. Ethical approval was obtained from the local authorities under the number 060916.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Desroziers, T., Prévot, G., Coulomb, A. et al. Hypomorphic pathogenic variant in SFTPB leads to adult pulmonary fibrosis. Eur J Hum Genet 31, 1083–1087 (2023). https://doi.org/10.1038/s41431-023-01413-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41431-023-01413-w
This article is cited by
-
2023 in the European Journal of Human Genetics
European Journal of Human Genetics (2024)
-
Why don’t we all use genomic testing?
European Journal of Human Genetics (2023)