Abstract
Diffuse large B-cell lymphoma (DLBCL) has been shown to be comprised of at least two prognostic entities, depending on its resemblance to normal germinal center or activated B cells, using global gene expression profiling. Also, the expression patterns of bcl-6, CD10 and IRF-4 (also known as MUM1) have been suggested as alternative means of identifying the germinal- and nongerminal center (activated B-cell like) groups. In the present study, we evaluated by immunohistochemistry the expression patterns of CD10, bcl-6, IRF-4 and bcl-2 in a large material of 161 DLBCL patients. Using two different approaches, patients with germinal center phenotype displayed a significantly better survival than the nongerminal center group. Positive staining for bcl-6 or CD10 predicted for superior survival, while expression of IRF-4 alone showed no association with prognosis. Furthermore, expression of bcl-2 was associated with worse event-free survival and overall survival. In a multivariate analysis, a high international prognostic index score (3–5), non-GC phenotype and bcl-2 were independent adverse prognostic factors. Here we confirm the prognostic importance of determining the germinal- or nongerminal center phenotype in patients with DLBCL.
Similar content being viewed by others
Main
Diffuse large B-cell lymphoma (DLBCL) is the most common type of B-cell lymphoma and accounts for about 40% of cases.1, 2 DLBCL is very heterogeneous on a molecular and clinical level,3, 4 which makes prognostication and decisions in treatment strategy difficult. The International Prognostic Index (IPI), based on clinical parameters, is currently the most important prognostic tool for survival prediction and choice of treatment.5 However, using cDNA microarrays it has been shown that DLBCLs could be divided into important subgroups with regard to prognosis with either germinal center B-cell like, activated B-cell like, or type 3 (a group of unclassified cases) gene expression profiles,6, 7, 8 where the germinal center B-cell like group show a significantly better survival compared to the activated B-cell like or the type 3 group. The germinal center B-cell like and activated B-cell like groups were initially identified according to their gene expression patterns resembling normal germinal center B-cells or activated B-cells, whereas the type 3 group has been poorly described.7 The activated B-cell like-group and the type 3 entities have later been grouped together as the nongerminal center group, since the type 3 group have similar outcome as the activated B-cell like-group.7 Recently, bcl-6, CD10 and IRF-4 have been shown to be differently expressed in the germinal center B-cell like and activated B-cell like groups (nongerminal center) using gene expression arrays as well as immunohistochemistry.6, 7, 8, 9 These three markers might successfully subdivide germinal- and nongerminal center DLBCL as shown by others.10 Both CD10 and bcl-6 are considered as germinal center markers while IRF-4 has been found expressed in plasma cells and a subset of cells in the apical light zone of the germinal center.11, 12 Other new prognostic markers have also been suggested in DLBCL such as p53 and bcl-2 expression;10, 13, 14, 15, 16, 17, 18 however, different studies have shown divergent results and the prognostic values of some of these markers is still not clear.19 In DLBCL, many of the patients have an earlier history of a low-grade malignant lymphoma, especially follicular lymphoma, and these cases have been shown to be more similar to the ancestral lymphoma type on a molecular level.20 Therefore, in this study, only de novo DLBCL cases from three University Hospitals in Sweden were included in the evaluation of the prognostic value of immunostainings for bcl-6, CD10, IRF-4 and bcl-2, in relation to IPI and survival in an independent material of DLBCL.
Materials and methods
Patients and Tumor Specimens
A total of 161 DLBCL patients (82 men and 79 women), diagnosed between 1984 and 2002 with a median age of 62 years (range 16–90) were included in the study. The patients were identified from the files of the Departments of Pathology at the Uppsala University Hospital (n=85), Umeå University Hospital (n=39) and Lund University Hospital (n=37), Sweden. Paraffin-embedded tumor biopsies from the time of diagnosis and a clinical follow-up were available for all patients. The pathology was reviewed by four of the authors (R-MA, CS, MD and GR) and confirmed to be de novo DLBCL according to the REAL and WHO classification.2, 21 Patients with primary CNS-lymphoma, post-transplant lymphoma or lymphoma related to AIDS were excluded.
The International Prognostic Index (IPI)5 was retrospectively evaluated in all patients. Owing to the retrospective analysis, all factors were not available for all patients. However, 156 of the 161 patients could be divided in two groups; a low-risk group with 0, 1 or 2 risk factors and a high-risk group with 3, 4 or 5 risk factors. The clinical characteristics of the patients are presented in Table 1. The patients were mainly treated with CHOP or CHOP-like regimens.
Immunohistochemistry
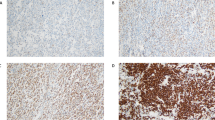
Formalin-fixed paraffin sections 4 μm thick were deparaffinized and rehydrated. Tissue sections for IRF-4 were antigen retrieved by incubation in 10 mM citrate buffer (pH 6.0) and by heat induction for 2 × 10 min. The goat anti-IRF-4 antibody (diluted 1/100, clone M17; Santa Cruz Biotechnology, Santa Cruz, CA, USA), biotinulated rabbit-anti-goat antibody (diluted 1/200; Dako, Glostrup, Denmark) and HRP coupled avidin–biotin complex (Dako) were added sequentially for 30 min at room temperature. Immunoreactivity was visualized with 3,3-diaminobenzidine and sections were counterstained with Mayer's hematoxylin. Mouse monoclonal bcl-2 (diluted 1/10, clone 124; Dako), bcl-6 (diluted 1/10, clone PG-B6p)(Dako) and CD10 (1/20, clone 56C6) (Ventana medical systems, Tucson, USA) antibody stainings were performed in a Ventana benchmark machine (Ventana). Buffer CC2 (Ventana) was used for antigen retrieval. Primary antibodies were incubated for 30 min and the DAB detection kit (Ventana) was used for the stainings. Normal goat and mouse sera (Dakopatts) were used as negative controls and sections of reactive tonsil as positive controls. The proportion of positively stained tumor cells was visually estimated by two pathologists (RA, MD). For each case, the hot-spot with the highest percentage of tumor cells was used for analysis. Cases were considered as positive for CD10, bcl-6 and IRF-4 if 30% or more of the tumor cells were positively stained by the respective antibodies in accordance with the study by Hans et al.10 In order to evaluate the choice of cutoff level in this material, cutoff levels of 10 and 20% were also tested for CD10, bcl-6 and IRF-4. For bcl-2, a cutoff level of 50% positive cells was used since the results were superior than that of/when using 30% as carried out by Hans et al, 20% positive cells was also tested as cut off limit but with inferior results. Two different strategies were employed in order to subclassify into a germinal- or nongerminal center phenotype using the results from the CD10, bcl-6 and IRF-4 stainings. Firstly, all cases were considered to be of a germinal center phenotype if both CD10 and bcl-6 were positive, whereas the remaining cases were considered as nongerminal center cases. This method was assigned the two marker model. Secondly, cases were classified as germinal center if CD10 alone or both CD10 and bcl-6 were positive, as described earlier by Hans et al.10 If CD10 was negative, but bcl-6 was positive, the IRF-4 staining determined the group: if IRF-4 was negative, a germinal center subtype was assigned and all other cases were considered as nongerminal center (Figure 1). This method was assigned the three-marker model.
Statistical Analysis
χ2 analysis was used to compare differences in proportions. Differences in age distributions between subgroups were analyzed with the Mann–Whitney U-test. Kaplan–Meier survival analysis and log-rank test were performed in order to study the prognostic significance of the markers used. Overall survival was calculated from the date of diagnosis until last follow-up or death. Patients alive and in remission were censored in those analyses. Event-free survival was calculated from date of diagnosis to death, progression or end of follow-up. Five-year survival was calculated from the Kaplan–Meier graphs, 10-year survival could not be determined for all parameters investigated. Probabilities of less than 0.05 were accepted as a significant value. In order to compare the prognostic importance of different variables, Cox proportional hazard multivariate analyses were performed. The Statistica 6.1 software (Stat Soft Inc., Tulsa, US) was used for all calculations.
Results
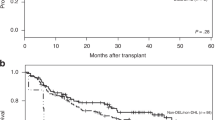
The 5- and 10-year event-free survival of all patients were 47 and 31%, respectively, and the corresponding figures for overall survival were 46 and 29%. Patients with 0, 1 or 2 risk factors (n=118) according to IPI showed a significantly better survival as compared to those with 3, 4 or 5 (n=50) factors (P<0.000001) (Figure 2). The 5-year overall survivals were 59 and 17%, respectively. For CD10, bcl-6 and IRF-4, cutoff levels of 30% rather than 10 and 20% were found to better discriminate between subgroups with different survival, whereas for bcl-2, cutoff levels of 50% showed superior results compared to 20 and 30% (data not shown).
Expression of CD10 was demonstrated in 35% of cases (56/161), bcl-6 in 48% (78/161), IRF-4 in 32% (51/161) and bcl-2 in 55% (89/161). The event-free survival and overall survival for the individual stainings are presented in Table 2. In brief, expression of bcl-6 was associated with a significantly better event-free survival and overall survival (P=0.000001 and 0.00003, respectively). Patients with positive staining for CD10 also displayed a better event-free survival and overall survival (P=0.007 and 0.04, respectively). Positive staining for bcl-2 was an adverse prognostic factor for event-free survival and overall survival (P=0.0006 and 0.03 respectively). No correlation was found between IRF-4 expression and survival.
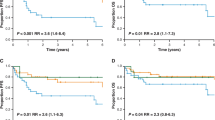
From the 157 cases on which stainings were performed, 82 (52%) were found to show a germinal center phenotype according to the three-marker model, whereas 75 (48%) were assigned to the nongerminal center group/phenotype. The germinal center group showed a significantly better event-free survival and overall survival than the nongerminal center group (P=0.00001 and 0.002, respectively) (Figure 3). Of the 82 cases with germinal center phenotype, 12 showed expression of IRF-4. This group showed similar event-free survival and overall survival compared with the IRF-4 negative cases in the germinal center group. In all, 15 patients with bcl-6 expression in the nongerminal center group had a significantly better event-free survival and overall survival than the bcl-6 negative cases (P=0.006 and 0.04, respectively).
When using the two-marker model, that is, concomitant expression of bcl-6 and CD10, a statistically significant survival difference was also found for both event-free survival and overall survival (P=0.007 and 0.009, respectively), although a lower proportion of the cases were assigned to the germinal center group (40 cases). There was no difference in any clinical parameter analyzed between the germinal- and nongerminal center group (Table 1) using either the two- or three-marker models for defining the germinal/nongerminal center groups.
When bcl-2 stainings were analyzed in the germinal- and nongerminal center group separately using the three-marker definition, the event-free survival differences were retained in both groups (data not shown), while overall survival was significantly inferior for bcl-2-positive patients in the germinal center group but not in the nongerminal center group. In this study, by adding bcl-2 to the scheme for the three-marker model no improvement of the results was evident. The impact of bcl-2 expression was also analyzed in the two groups with patients with IPI 0, 1 or 2 and IPI >2 separately, and a clear difference in event-free survival and overall survival was revealed between these groups; patients in the group with a low IPI (0, 1 or 2) displaying a positive bcl-2 staining, had a significantly worse event-free survival (P=0.0006) and overall survival (P=0.02) than patients negative for bcl-2, whereas in patients with a high IPI score (>2) no difference was shown for event-free survival and overall survival (P=0.12 and 0.30, respectively).
When combining the germinal center phenotype with IPI, four prognostic groups were revealed, one with a germinal center phenotype and IPI≤2 (n=50), one with a germinal center phenotype and IPI>2 (n=26), a nongerminal center phenotype and IPI≤2 (n=49), and nongerminal center phenotype and IPI>2 (n=28) (Figure 4). Best survival was found in the germinal center IPI≤2 group and worst survival in the groups with IPI>2 (P<0.000001). Furthermore, a very favorable group (29 patients) could be identified with IPI 0, 1 or 2, germinal center phenotype and negative bcl-2 staining. The 5-year event-free survival and overall survival in that group was approximately 85%.
In a multivariate analysis including IPI (0–2 vs 3–5), germinal vs nongerminal center (three-marker model) and bcl-2-positive vs negative, all factors were significantly related to event-free survival (P<0.00001, 0.0004 and 0.00008, respectively) and overall survival (P<0.0000001, 0.02 and 0.01, respectively). All included factors, that is, IPI, germinal center-phenotype and bcl-2 were thus independent prognostic factors in the prediction of outcome.
Discussion
The clinical presentation and course of DLBCL is very heterogeneous and a number of biological prognostic factors have been analyzed in an effort to improve the subdivision of the disease, but with inconsistent results.7, 8, 13, 14, 16, 17 In recent years, knowledge about DLBCL has increased dramatically in light of the repeated finding of a germinal center and a nongerminal center group (ie the activated B-cell like and type 3 groups) using gene expression profiles.6, 7, 8 This has made it possible to study new risk factors in more biologically distinct subgroups of DLBCL. However, gene expression profiles are difficult to incorporate in routine diagnosis and the preferred approach would be to supplant gene expression profiling with immunohistochemistry to identify the same groups. This approach was applied by Hans et al10 who revealed that conventional immunohistochemistry could give similar results concerning prognosis using protein expression patterns for selected markers, that is, bcl-6, CD10 and IRF-4. In the present study, we confirmed those results in an independent material by showing that patients with germinal center phenotype had a significantly better outcome compared to patients with the nongerminal center phenotype. We could also show that IPI, germinal center phenotype and bcl-2 are important independent prognostic factors in DLBCL.
The most common definition in the literature of the germinal center-group of DLBCL is concomitant-positive staining for bcl-6 and CD10.15, 19, 22 Using coexpression of CD10 and bcl-6 to determine the germinal center phenotype has been shown to be predictive for overall survival14 although some of the cases positive for only one of these two markers may have been misclassified as nongerminal center. To avoid this problem Hans et al10 employed a model including the usage of markers specific for both germinal center (bcl-6/CD10) and nongerminal center (IRF-4). Interestingly, using this three-marker model the initial results obtained by the microarray analysis could be reproduced in 71 and 88% of the germinal center B-cell like and nongerminal center B-cell like cases (ie the activated B-cell like and the type 3 group), respectively. It was also noted that this approach using immunostaining may give a better prediction for survival than the expression profiling.10 Accordingly, our results showed that the three-marker model gave an improved subdivision of DLBCL than just using bcl-6 and CD10 expression (the two-marker model), especially considering the fact that the three-marker model defines a larger group of germinal center patients. In contrast, an earlier study that used CD10, bcl-6, IRF-4 and CD138 to classify into germinal center CD10+, germinal center CD10−, postgerminal center and plasmablastic subgroups13 found no difference in survival between the germinal- and nongerminal center cases. However, the difference in subgrouping strategy and the fewer patients included might to some extent explain the divergent results. Our findings thus confirm the prognostic usage of the three-marker model to subdivide the germinal- and nongerminal center type of DLBCL.
In order to assess the question if the three-marker method could be used as an independent complement to the IPI score a multivariate analysis were performed and they were shown to be independent factors. We also analyzed the impact of GC-phenotype according to the three-marker model in patients with a low IPI (0-2), and a high IPI (3-5) separately. Interestingly, the GC phenotype was only of importance in the group with IPI 0-2. This differs from the earlier study,10 where there were no differences between the groups. In both cases few patients were included, which may partly explain the discrepancy, but differences in antibodies used (polyclonal vs monoclonal bcl-6 and IRF-4) may also influence the results.
Varying cutoff levels for determining positive stainings for bcl-6, CD10 and bcl-2 have been applied in different studies to study prognostic impact, which could be one explanation for the divergent results in earlier studies. The most common level for bcl-6 and CD10 is 10–30% and for bcl-2 is 10–50%.10, 14, 18, 19, 23, 24, 25 In the present study, we used the cutoff levels chosen by Hans et al with 30% for bcl-6, CD10 and IRF-4. We thereafter applied other cutoff levels to analyze the best discriminator for prognosis but other cutoff levels were inferior and in the present material the levels chosen by Hans et al seemed optimal, with the exception of bcl-2, where 50% was shown to be better. It should however be noted, that the pattern of staining for bcl-2 shows a continuous distribution from 0 to 100% positive cells which makes the selection of a cutoff value difficult and thus should be further evaluated.
With the exception of germinal center and IPI, bcl-6 alone seemed to be an equally good prognostic factor in the present study. Expression of bcl-6 has been suggested as a prognostic marker in DLBCL23, 24 but some studies have been in contradiction.13, 19, 26 These differences may partly be explained by the differences in the cutoff value (10–30%) for a positive staining, where 10% is most commonly employed, but as shown by others, this level might be too low to subdivide DLBCL into manageable subgroups with similar numbers of patients.10 Also, differences in staining techniques may be of importance, for instance in one study using the EnVision method and a cutoff of 10%, as much as 97% of cases were found positive for bcl-6,26 which indicates a very high sensitivity for detection of bcl-6. Furthermore, others have used the chromosomal locus 3q2724 as a marker for aberrant bcl-6 expression; however, this may give a false reflection of bcl-6 expression since other mechanisms also result in increased bcl-6 expression, such as mutations in the bcl-6 binding sites of bcl-6.27 We therefore believe that it is important to measure the expression of bcl-6 by immunohistochemistry to avoid misclassification. The favorable prognostic value of bcl-6 may be due to discrimination between subgroups with different clinical outcomes but does not necessarily imply that bcl-6 expression is of any advantage for the patient. However, for treatment of DLBCL, bcl-6 may be an important therapeutic target.27, 28 Using bcl-6 only as a prognostic marker may give divergent results between studies depending on methods used and we believe that a model including more markers, as in the three-marker model is preferable.
CD10 is a membrane metalloproteinase which is found in a variety of lymphoid cells as well as in stromal and epithelial cells.19, 29 It has also been used as a marker for the germinal center in reactive lymphoid tissue as well as in lymphomas.15, 19, 29, 30, 31 The prognostic significance of CD10 expression has been evaluated in many studies with controversial results. In some studies a longer survival was found for patients with tumors that expressed CD10,10, 32 but both similar and worse survival have also been reported.13, 33, 34 In our study, CD10 predicted prognosis especially well in the group with a low IPI which is in accordance with the study by Ohshima et al.32 Since most of CD10-positive cases also expressed bcl-6 (84%) it might be difficult to know how this latter marker influences the prognostic value of CD10.
IRF-4 is a transcriptional regulator thought to be involved in activation of T cells, lineage commitment of lymphocytes and in Fas-dependent apoptosis,35 as well as being a marker for postgerminal center stage. Using the three-marker model, where bcl-6 and CD10 are germinal center markers and IRF-4 a postgerminal center antigen, the prognostic usage of IRF-4 is evident in our study. However, IRF-4 does not alone give any prognostic value, which is in contrast to Hans et al10 who found a worse outcome in IRF-4 positive DLBCL. Our findings are supported by Colomo et al13 who also did not find any association with clinical outcome for IRF-4 but where IRF-4 expression was related to immunoblastic morphology and primary nodal presentation.13 The differences in outcome prediction using IRF-4 could partly be explained by a higher proportion of patients with more aggressive disease in this study.10 Another reason may be the usage of a monoclonal antibody by Hans et al, which may be more specific. The use of a polyclonal antibody as in our case may render more false positives. However, studies using polyclonal antibodies for IRF-4, have shown predictive value in a univariate analysis but was not significant as a independent risk factor.9 This discrepancy may be explained by fewer included patients but also a higher frequency of extranodal cases, 64% compared to 36% in this study. The frequency of IRF-4-positive cases within the germinal center group is similar in our study and the study undertaken by Hans et al. The classifications of these cases are not clear-cut since they express both germinal- and postgerminal center markers.
Bcl-2 expression has been shown to be an adverse prognostic factor in earlier studies as well as in large-scale clinical trials, both alone and in conjunction with other factors.10, 14, 36, 37, 38, 39 However, its importance as a prognostic marker is controversial since many studies have not found any statistically significant difference in overall survival.40, 41, 42 In the present study, we confirmed the prognostic importance of bcl-2 expression, which showed a similar predictive capacity in both the germinal- and nongerminal center group. Other studies have found a shorter time to relapse in patients with high bcl-2 expression and consequently found it to be more important for event-free survival than overall survival.40, 42, 43 In comparison with IPI, the bcl-2 expression was only of importance in the good prognostic risk group with IPI 0,1 or 2 and since patients with a high IPI show a lower rate of complete response and a higher rate of relapse after complete response,5 it indicates that factors other than bcl-2 expression are mainly responsible for the worse event-free survival in these patients.
In summary, we have been able to confirm the prognostic impact of assessing the germinal center phenotype of DLBCL using a three-marker model as well as the bcl-6, CD10 and bcl-2 expression in a large independent material. This might be the first step to a deeper understanding of the biology of the heterogeneous group of DLBCL. Immunohistochemical staining for the three-marker models including bcl-6, CD10 and IRF-4 as well as bcl-2 staining should be further evaluated for a possible incorporation in the routine evaluation of all new DLBCL cases.
References
Coiffier B . Diffuse large cell lymphoma. Curr Opin Oncol 2001;13:325–334.
Gatter KC, Warnke RA . Diffuse large B-cell lymphoma. In: Jaffe ES, Harris NL, Stein H, Vardiman JW (eds). Pathology and Genetics of Tumours of Haematopoetic and Lymphoid Tissues. International Agency for Research on Cancer (IARC) Press: Lyon, France, 2001, pp 171–174.
Berglund M, Enblad G, Flordal E, et al. Chromosomal imbalances in diffuse large B-cell lymphoma detected by comparative genomic hybridization. Mod Pathol 2002;15:807–816.
Pileri SA, Dirnhofer S, Went P, et al. Diffuse large B-cell lymphoma: one or more entities? Present controversies and possible tools for its subclassification. Histopathology 2002;41:482–509.
A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med 1993;329:987–994.
Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000;403:503–511.
Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 2002;346:1937–1947.
Wright G, Tan B, Rosenwald A, et al. A gene expression-based method to diagnose clinically distinct subgroups of diffuse large B cell lymphoma. Proc Natl Acad Sci USA 2003;100:9991–9996.
Chang CC, McClintock S, Cleveland RP, et al. Immunohistochemical expression patterns of germinal center and activation B-cell markers correlate with prognosis in diffuse large B-cell lymphoma. Am J Surg Pathol 2004;28:464–470.
Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2003;103:275–282.
Natkunam Y, Warnke RA, Montgomery K, et al. Analysis of MUM1/IRF4 protein expression using tissue microarrays and immunohistochemistry. Mod Pathol 2001;14:686–694.
Falini B, Fizzotti M, Pucciarini A, et al. A monoclonal antibody (MUM1p) detects expression of the MUM1/IRF4 protein in a subset of germinal center B cells, plasma cells, and activated T cells. Blood 2000;95:2084–2092.
Colomo L, Lopez-Guillermo A, Perales M, et al. Clinical impact of the differentiation profile assessed by immunophenotyping in patients with diffuse large B-cell lymphoma. Blood 2003;101:78–84.
Barrans SL, Carter I, Owen RG, et al. Germinal center phenotype and bcl-2 expression combined with the International Prognostic Index improves patient risk stratification in diffuse large B-cell lymphoma. Blood 2002;99:1136–1143.
Ree HJ, Yang WI, Kim CW, et al. Coexpression of Bcl-6 and CD10 in diffuse large B-cell lymphomas: significance of Bcl-6 expression patterns in identifying germinal center B-cell lymphoma. Hum Pathol 2001;32:954–962.
Sohn SK, Jung JT, Kim DH, et al. Prognostic significance of bcl-2, bax, and p53 expression in diffuse large B-cell lymphoma. Am J Hematol 2003;73:101–107.
Leroy K, Haioun C, Lepage E, et al. p53 gene mutations are associated with poor survival in low and low-intermediate risk diffuse large B-cell lymphomas. Ann Oncol 2002;13:1108–1115.
Zettl A, Meister S, Katzenberger T, et al. Immunohistochemical analysis of B-cell lymphoma using tissue microarrays identifies particular phenotypic profiles of B-cell lymphomas. Histopathology 2003;43:209–219.
De Leval L, Harris NL . Variability in immunophenotype in diffuse large B-cell lymphoma and its clinical relevance. Histopathology 2003;43:509–528.
Lossos IS, Alizadeh AA, Diehn M, et al. Transformation of follicular lymphoma to diffuse large-cell lymphoma: alternative patterns with increased or decreased expression of c-myc and its regulated genes. Proc Natl Acad Sci USA 2002;99:8886–8891.
Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 1994;84:1361–1392.
Ree HJ, Ohsima K, Aozasa K, et al. Detection of germinal center B-cell lymphoma in archival specimens: critical evaluation of Bcl-6 protein expression in diffuse large B-cell lymphoma of the tonsil. Hum Pathol 2003;34:610–616.
Lossos IS, Jones CD, Warnke R, et al. Expression of a single gene, BCL-6, strongly predicts survival in patients with diffuse large B-cell lymphoma. Blood 2001;98:945–951.
Barrans SL, O’Connor SJ, Evans PA, et al. Rearrangement of the BCL6 locus at 3q27 is an independent poor prognostic factor in nodal diffuse large B-cell lymphoma. Br J Haematol 2002;117:322–332.
Saez AI, Saez AJ, Artiga MJ, et al. Building an outcome predictor model for diffuse large B-cell lymphoma. Am J Pathol 2004;164:613–622.
Linderoth J, Jerkeman M, Cavallin-Stahl E, et al. Immunohistochemical expression of CD23 and CD40 may identify prognostically favorable subgroups of diffuse large B-cell lymphoma: a Nordic Lymphoma Group Study. Clin Cancer Res 2003;9:722–728.
Pasqualucci L, Migliazza A, Basso K, et al. Mutations of the BCL6 proto-oncogene disrupt its negative autoregulation in diffuse large B-cell lymphoma. Blood 2003;101:2914–2923.
Bereshchenko OR, Gu W, Dalla-Favera R . Acetylation inactivates the transcriptional repressor BCL6. Nat Genet 2002;32:606–613.
Dogan A, Bagdi E, Munson P, et al. CD10 and BCL-6 expression in paraffin sections of normal lymphoid tissue and B-cell lymphomas. Am J Surg Pathol 2000;24:846–852.
Bai M, Agnantis NJ, Skyrlas A, et al. Increased expression of the bcl6 and CD10 proteins is associated with increased apoptosis and proliferation in diffuse large B-cell lymphomas. Mod Pathol 2003;16:471–480.
Bilalovic N, Blystad AK, Golouh R, et al. Expression of bcl-6 and CD10 protein is associated with longer overall survival and time to treatment failure in follicular lymphoma. Am J Clin Pathol 2004;121:34–42.
Ohshima K, Kawasaki C, Muta H, et al. CD10 and Bcl10 expression in diffuse large B-cell lymphoma: CD10 is a marker of improved prognosis. Histopathology 2001;39:156–162.
Uherova P, Ross CW, Schnitzer B, et al. The clinical significance of CD10 antigen expression in diffuse large B-cell lymphoma. Am J Clin Pathol 2001;115:582–588.
Harada S, Suzuki R, Uehira K, et al. Molecular and immunological dissection of diffuse large B cell lymphoma: CD5+, and CD5− with CD10+ groups may constitute clinically relevant subtypes. Leukemia 1999;13:1441–1447.
Fanzo JC, Hu CM, Jang SY, et al. Regulation of lymphocyte apoptosis by interferon regulatory factor 4 (IRF-4). J Exp Med 2003;197:303–314.
Rantanen S, Monni O, Joensuu H, et al. Causes and consequences of BCL2 overexpression in diffuse large B-cell lymphoma. Leuk Lymphoma 2001;42:1089–1098.
Monni O, Franssila K, Joensuu H, et al. BCL2 overexpression in diffuse large B-cell lymphoma. Leuk Lymphoma 1999;34:45–52.
Kramer MH, Hermans J, Wijburg E, et al. Clinical relevance of BCL2, BCL6, and MYC rearrangements in diffuse large B-cell lymphoma. Blood 1998;92:3152–3162.
Jerkeman M . Aggressive lymphoma.. Department of Oncology, Jubileum Institute, Lund, Lund University, 2000.
Hill ME, MacLennan KA, Cunningham DC, et al. Prognostic significance of BCL-2 expression and bcl-2 major breakpoint region rearrangement in diffuse large cell non-Hodgkin's lymphoma: a British National Lymphoma Investigation Study. Blood 1996;88:1046–1051.
Zhang A, Ohshima K, Sato K, et al. Prognostic clinicopathologic factors, including immunologic expression in diffuse large B-cell lymphomas. Pathol Int 1999;49:1043–1052.
Sanchez E, Chacon I, Plaza MM, et al. Clinical outcome in diffuse large B-cell lymphoma is dependent on the relationship between different cell-cycle regulator proteins. J Clin Oncol 1998;16:1931–1939.
Gascoyne RD, Adomat SA, Krajewski S, et al. Prognostic significance of Bcl-2 protein expression and Bcl-2 gene rearrangement in diffuse aggressive non-Hodgkin's lymphoma. Blood 1997;90:244–251.
Acknowledgements
This study was supported by grants from the Swedish Cancer Society, the Lions Cancer research foundation in Uppsala and the Research foundation of the Department of Oncology, Uppsala University Hospital, Sweden.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berglund, M., Thunberg, U., Amini, RM. et al. Evaluation of immunophenotype in diffuse large B-cell lymphoma and its impact on prognosis. Mod Pathol 18, 1113–1120 (2005). https://doi.org/10.1038/modpathol.3800396
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800396
Keywords
This article is cited by
-
Treatment outcome and prognostic factors in PCNSL
Diagnostic Pathology (2019)
-
A modular transcriptome map of mature B cell lymphomas
Genome Medicine (2019)
-
BCL2 expression is associated with a poor prognosis independent of cellular origin in primary central nervous system diffuse large B-cell lymphoma
Journal of Neuro-Oncology (2018)
-
Clinical significance and functional validation of inorganic pyrophosphatase in diffuse large B cell lymphoma in humans
Cytotechnology (2018)
-
Current Perspectives in Genetics of “Double-Hit” Lymphoma with Possible Clinical Implications
Cell Biochemistry and Biophysics (2014)