Abstract
The prognosis for small cell carcinoma of the urinary bladder is poor, and strategies for improved therapy are needed. Targeted therapy against the c-kit proto-oncogene has been successful in the management of gastrointestinal stromal tumor. We investigated the expression of c-kit in 52 cases of small cell carcinoma of the urinary bladder. Specimens with more than 10% of cells demonstrating strong membrane staining were considered to have positive immunostaining for c-kit. c-kit expression was detected in 21 of 52 specimens from these patients. Among the 21 specimens, seven had less than 10% staining, and were considered to be negative. Nine had 11–50% staining, and five had more than 50% staining. Overall, 14 of 52 (27%) small cell carcinomas of the urinary bladder were positive for c-kit expression. During a median follow-up of 11 months, 60% of the patients died of bladder cancer. No association was found between c-kit expression and survival or other clinicopathologic parameters. Five-year cancer-specific survivals for c-kit-positive and c-kit-negative tumors were 9 and 15%, respectively (P=0.36). A significant proportion (27%) of small cell carcinomas of the urinary bladder expressed c-kit, suggesting that it may prove useful as a therapeutic target in small cell carcinoma of the urinary bladder.
Similar content being viewed by others
Main
Small cell carcinoma of the urinary bladder is a distinct histological and biologic disease entity with an aggressive clinical course, poor prognosis, and an average life expectancy of only a few months.1, 2, 3 Consequently, strategies for improved therapy of this highly lethal cancer are needed. Therapy directed at tumor-specific targets has been successful in improving survival in some cancers. The proto-oncogene c-kit encodes a transmembrane tyrosine kinase receptor c-kit (also called CD117), which has been shown to be involved in hematopoiesis and carcinogenesis.4, 5 Overexpression of c-kit has been detected in several malignancies, including gastrointestinal stromal tumor, carcinoma of breast, and small cell carcinoma of the lung.6, 7, 8 Gastrointestinal stromal tumor has been successfully treated with therapy targeted against c-kit.9 We studied the expression of c-kit in a large series of small cell carcinomas of the urinary bladder and correlated the findings with other clinicopathologic parameters.
Materials and methods
Patients
In total, 52 patients with small cell carcinoma of the urinary bladder were identified from Indiana University (Indianapolis, IN, USA), Case Western Reserve University (Cleveland, OH, USA), Northwestern University (Chicago, IL, USA), University of Chicago (Chicago, IL, USA), and Cordoba University (Cordoba, Spain). The medical records were reviewed. Formalin-fixed paraffin-embedded tissue specimens were available for all cases. Sections stained with hematoxylin and eosin were examined. All tumors fulfilled the criteria established for small cell carcinoma by the WHO classification system.10 The diagnosis was further supported by immunohistochemical stains and, in some cases, electron microscopic examination. Pathologic staging was performed according to the 2002 TNM (tumor, lymph nodes, and metastasis) classification system.11 This research was approved by the Institutional Review Boards in accordance with the Institutional Committee for the Protection of Human Subjects.
Immunohistochemistry
Immunohistochemical staining was performed with the avidin–biotin complex method of Hsu et al.12 In brief, serial 5 μm-thick sections of formalin-fixed, paraffin-embedded tissue samples were used for the studies. Slides were deparaffinized twice in xylene for 5 min and rehydrated through graded ethanol solutions to distilled water. Antigen retrieval was carried out by heating sections in citrate buffer (DAKO Target Retrieval Solution S1699, DAKO Corporation, Carpintera, CA, USA) for 15 min. Endogenous peroxidase activity was inactivated by incubation in 3% H2O2 for 15 min. Nonspecific binding sites were blocked using Protein Block (DAKO) for 20 min. Tissue sections were then incubated with the purified rabbit polyclonal antibody against c-kit (IgG, 1:50 dilution; Oncogene, Boston, MA, USA) for 30 min at room temperature, followed by biotinylated secondary antibody (DAKO) and peroxidase-labeled streptavidin. 3,3-Diaminobenzidine was used as the chromogen in the presence of hydrogen peroxide. Sections from gastrointestinal stromal tumors were used as positive controls. Negative controls were performed using blocking serum in place of primary antibody. Positive and negative controls were run in parallel with each batch, and appropriate results were obtained.
The expression of c-kit was graded as previously described.7 The entire section was scanned at low magnification, and hot spots were preferentially scored. At least 1000 cells were analyzed. The cutoff value of 10% for positive staining is based on the criteria used in the literature for c-kit staining,7 and other tumor markers, such as Her-2/neu in breast cancers.13, 14 The authors recognize that there is a need for standardization of c-kit immunostaining evaluation in bladder tumors.
Statistical Analysis
All statistical tests were two-sided, with a P-value of 0.05 or less considered to be statistically significant. SAS version 8.2 was used for the statistical analysis. SPLUS was used for Kaplan–Meier survival curves. Fisher's exact test for differences in frequencies was used for analyzing the c-kit expression and survival and other clinicopathologic parameters.
Results
Totally, 52 patients were analyzed. The male to female ratio was 4:1. The patients' ages ranged from 36 to 85 years (mean, 67 years). All patients except one had advanced disease (T2 or above) at presentation. Pathologic stage was T1 (1 patient, 2%), T2 (26 patients, 50%), T3 (22 patients, 42%), and T4 (three patients, 6%), respectively. Among patients with known clinical history, 57% (21/37) of patients had lymph node metastases at the time of radical cystectomy; four had distant metastases at presentation. Four patients underwent transurethral resection of bladder tumor. Overall, 55% received chemotherapy, while only 27% received radiation.
Expression of c-kit was evaluated by immunohistochemistry in specimens from 52 patients. The staining was predominantly located on the cell membrane (Figure 1), in keeping with the fact that c-kit is a membrane-bound tyrosine kinase receptor. The normal urothelium and stromal cells were negative for c-kit immunostaining. The expression of c-kit was graded as previously described.7 Specimens were considered to be positive when more than 10% of tumor cells showed positive immunostaining. Of the 52 specimens, 38 samples (73%) were negative (31 samples with no staining, seven with less than 10% staining). In all, 14 samples (27%) were positive for c-kit expression (nine between 11 and 50%, five with more than 50% staining).
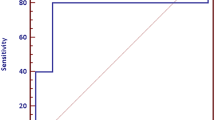
There was no correlation between c-kit expression and survival (P=0.36) (Figure 2). For c-kit positive tumors, 2- and 5-year cancer-specific survivals were 34 and 9%, respectively. For c-kit negative tumors, 2- and 5-year cancer-specific survivals were 41 and 15%, respectively. There was no correlation of c-kit expression with other clinicopathological characteristics including age (P=0.79), gender (P=0.41), history of smoking (P=0.58), clinical stage (P=0.20), pathologic T stage (P=0.51), lymph node metastasis (P=0.41), and distant metastasis (P=0.99).
Discussion
Overexpression of c-kit has been detected in 28–78% of cases of small cell carcinoma of the lung.7, 8, 15 Mutation of c-kit is uncommon, as detected by polymerase chain reaction-single-strand conformational polymorphism.16 There have been conflicting reports on the prognostic significance of c-kit expression. Potti et al7 observed a significantly worse prognosis in small cell lung carcinoma patients with c-kit overexpression, whereas other investigators have noted a trend toward better prognosis in such patients.17 In this study, we found that a significant proportion (27%) of small cell carcinomas of the urinary bladder had positive c-kit expression, raising the question of whether therapy targeted against c-kit may be beneficial for some patients with small cell carcinoma of the urinary bladder.
The c-kit gene encodes a tyrosine kinase receptor related to platelet-derived growth factor (PDGF)/colony-stimulating factor 1 (CSF-1).18 The c-kit pathway has been shown to be involved in a number of physiological and pathological processes, including hematopoiesis, spermatogenesis, melanogenesis, and oncogenesis.4, 15 Two mechanisms of c-kit activation exist: autocrine and/or paracrine activation by its ligands; and ligand-independent activation by mutation. Ligand-dependent activation of c-kit depends on the engagement of its ligand, the stem cell factor. The binding of c-kit by stem cell factor induces phosphorylation and activation of the c-kit signal transduction pathway. This type of activation occurs during some normal physiological processes, and during oncogenesis of some cancers.8, 19 It has been shown that some tumor cells express both c-kit and stem cell factor which appears to be capable of protecting the cells against apoptosis.19 Ligand-independent constitutive activation of c-kit involves mutations, mainly in exon 11, the region between the transmembrane and tyrosine kinase domains. c-kit protein derived from mutation is constitutively activated without engagement by its ligand, stem cell factor. Stable transfection of mutant c-kit complementary DNA has been shown to induce malignant transformation, suggesting that the mutations contribute to tumor development.20 Mutation of c-kit is common in gastrointestinal stromal tumor,20 whereas mutation is uncommon in small cell carcinoma of the lung and colon.16, 21 It has been suggested that c-kit expression does not accurately identify responders/nonresponders to STI-571 therapy for gastrointestinal stromal tumors. The presence or absence of activating mutations of c-kit is most predictive of clinical response.22 c-kit expression does not always correlate with the presence of activating mutation.16
STI-571 (Gleevec, Novartis, Basel, Switzerland), inhibiting c-kit phosphorylation and its subsequent activation, has been utilized in the successful treatment of gastrointestinal stromal tumor.9 The role of STI-571 in the treatment of other cancers is not well established. Whether the encouraging results of STI-571 treatment on gastrointestinal stromal tumor can be translated to small cell carcinoma of the urinary bladder is uncertain. STI-571 inhibits the growth of Ewing's sarcoma cell lines; the degree of inhibition correlates with the levels of c-kit expression. Nevertheless, the inhibition is less pronounced than in gastrointestinal stromal tumor, and high doses of STI-571 are required to accomplish inhibition.23 At the present time, there are no in vitro or in vivo models available to test the effectiveness of target therapy using STI-571 in small cell carcinoma of the urinary bladder. In small cell lung carcinoma patients treated with first-line chemotherapy, the expression of c-kit is lost in about half of the patients whose cancers expressed c-kit prior to chemotherapy.24
There is currently no randomized clinical trial for the management of small cell carcinoma of the urinary bladder. Current experience in the treatment of small cell carcinoma of the urinary bladder is based on anecdotal reports, some published more than a decade ago. Considering the generally poor prognosis of small cell carcinoma of the urinary bladder, it may be reasonable to consider the therapeutic use of STI-571 in patients with c-kit-positive tumors.
References
Grignon DJ, Ro JY, Ayala AG, et al. Small cell carcinoma of the urinary bladder. A clinicopathologic analysis of 22 cases. Cancer 1992;69:527–536.
Cheng L, Pan CX, Yang XJ, et al. Small cell carcinoma of the urinary bladder: a clinicopathologic analysis of 64 patients. Cancer 2004;101:957–962.
Mills SE, Wolfe III JT, Weiss MA, et al. Small cell undifferentiated carcinoma of the urinary bladder. A light-microscopic, immunocytochemical, and ultrastructural study of 12 cases. Am J Surg Pathol 1987;11:606–617.
Natali PG, Nicotra MR, Sures I, et al. Expression of c-kit receptor in normal and transformed human nonlymphoid tissues. Cancer Res 1992;52:6139–6143.
Pietsch T, Nicotra MR, Fraioli R, et al. Expression of the c-Kit receptor and its ligand SCF in non-small-cell lung carcinomas. Int J Cancer 1998;75:171–175.
Demetri GD . Targeting c-kit mutations in solid tumors: scientific rationale and novel therapeutic options. Semin Oncol 2001;28:19–26.
Potti A, Moazzam N, Ramar K, et al. CD117 (c-KIT) overexpression in patients with extensive-stage small-cell lung carcinoma. Ann Oncol 2003;14:894–897.
DiPaola RS, Kuczynski WI, Onodera K, et al. Evidence for a functional kit receptor in melanoma, breast, and lung carcinoma cells. Cancer Gene Ther 1997;4:176–182.
Joensuu H, Roberts PJ, Sarlomo-Rikala M, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med 2001;344:1052–1056.
Eble JN, Sauter G, Epstein JI, et al. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. IARC Press: Lyon, 2004.
Greene F, Page D, Fleming I, et al. AJCC Cancer Staging Manual, 6th ed, Springer-Verlag, New York, NY, 2002.
Hsu SM, Raine L, Fanger H . Use of avidin–biotin–peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 1981;29:577–580.
Peiro G, Mayr D, Hillemanns P, et al. Analysis of HER-2/neu amplification in endometrial carcinoma by chromogenic in situ hybridization. Correlation with fluorescence in situ hybridization, HER-2/neu, p53 and Ki-67 protein expression, and outcome. Mod Pathol 2004;17:277–287.
Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 1999;17:2639–2648.
Ullrich A, Schlessinger J . Signal transduction by receptors with tyrosine kinase activity. Cell 1990;61:203–212.
Burger H, den Bakker MA, Stoter G, et al. Lack of c-kit exon 11 activating mutations in c-KIT/CD117-positive SCLC tumour specimens. Eur J Cancer 2003;39:793–799.
Micke P, Basrai M, Faldum A, et al. Characterization of c-kit expression in small cell lung cancer: prognostic and therapeutic implications. Clin Cancer Res 2003;9:188–194.
Yarden Y, Kuang WJ, Yang-Feng T, et al. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J 1987;6:3341–3351.
Krystal GW, Hines SJ, Organ CP . Autocrine growth of small cell lung cancer mediated by coexpression of c-kit and stem cell factor. Cancer Res 1996;56:370–376.
Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998;279:577–580.
Akintola-Ogunremi O, Pfeifer JD, Tan BR, et al. Analysis of protein expression and gene mutation of c-kit in colorectal neuroendocrine carcinomas. Am J Surg Pathol 2003;27:1551–1558.
Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 2003;21:4342–4349.
Scotlandi K, Manara MC, Strammiello R, et al. C-kit receptor expression in Ewing's sarcoma: lack of prognostic value but therapeutic targeting opportunities in appropriate conditions. J Clin Oncol 2003;21:1952–1960.
Rossi G, Cavazza A, Marchioni A, et al. Kit expression in small cell carcinomas of the lung: effects of chemotherapy. Mod Pathol 2003;16:1041–1047.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pan, CX., Yang, X., Lopez-Beltran, A. et al. c-kit Expression in small cell carcinoma of the urinary bladder: prognostic and therapeutic implications. Mod Pathol 18, 320–323 (2005). https://doi.org/10.1038/modpathol.3800318
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800318
Keywords
This article is cited by
-
Immunohistochemical expression of CD117/KIT, HER2, and Erβ in schistosomal and non-schistosomal urothelial carcinoma of Egyptian patients
International Urology and Nephrology (2023)
-
Role of tyrosine kinases in bladder cancer progression: an overview
Cell Communication and Signaling (2020)
-
Neuroendocrine carcinoma of the urinary bladder: a retrospective study of CT findings
Abdominal Imaging (2013)
-
A rare bladder cancer - small cell carcinoma: review and update
Orphanet Journal of Rare Diseases (2011)