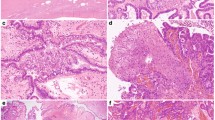
Abstract
K-RAS mutations are the most frequent molecular genetic alteration in serous ovarian tumors of borderline malignancy (SBOT). The pathogenesis of associated contralateral tumors and extraovarian implants and Müllerian inclusion cysts is obscure. We hypothesized that the comparison of K-RAS mutations in these lesions might help to distinguish multifocal from metastatic foci. Eight cases of SBOT with known K-RAS mutation (RAS+) and two cases without mutation (RAS−) were analyzed for comparison. DNA was extracted from multiple samples of 58 paraffin-embedded and laser-microdissected ovarian and extraovarian lesions (10 ovarian borderline tumors, 8 contralateral tumors, 25 implants, 15 inclusion cysts; total: 97 samples). K-RAS exon 1 was amplified by PCR and analyzed by denaturing gradient gel electrophoresis and cycle sequencing. In 12 of 14 SBOT and in 2 of 2 extraovarian implants, the K-RAS mutation could be found in different areas of the same lesion, indicating monoclonality. All RAS+ ovarian borderline tumors with contralateral tumors (six of six) harbored an identical mutation in both ovaries (in one case, a separate surface borderline tumor component contained a different mutation in addition). In 4 of 5 RAS+ ovarian tumors with extraovarian lesions, RAS mutations were also found in implants (15/21 implants [71%]) and more rarely in inclusion cysts (3 of 12 lesions [25%]). These extraovarian mutations were always identical to the one in the ovary (18 of 18 [100%]). Regarding the contralateral and extraovarian lesions of the two RAS− SBOT, only one extraovarian implant contained a RAS mutation. The demonstration of K-RAS mutations in Müllerian inclusion cysts and implants of SBOT suggests that K-RAS mutations represent a pivotal event during neoplastic transformation of ovarian and extraovarian serous epithelium. Considering our observations, the two putative pathogenetic mechanisms for the development of implants and endosalpingiosis—multifocal tumorigenesis and spread from the ovarian primary tumor—seem to coexist.
Similar content being viewed by others
Introduction
Serous borderline tumors of the ovary (SBOT), also called tumors of low malignant potential, are characterized by increased epithelial proliferation and structural as well as cytological atypia that distinguishes these tumors from serous cystadenomas of the ovary. In contrast to serous carcinomas, borderline tumors are noninvasive neoplasms. However, 30 to 40% of patients with SBOT have bilateral or multifocal lesions at the time of diagnosis (Chambers et al, 1988; Leake et al, 1992). These foci compose a spectrum from clearly benign Müllerian inclusion cysts to so-called implants with strong resemblance to the ovarian tumors. Implants are subclassified into noninvasive and invasive. Presence of invasive serous implants is associated with poor prognosis (Kennedy and Hart, 1996; Kurman and Trimble, 1993). The optimal treatment of patients with extraovarian lesions of serous borderline tumors is the topic of an ongoing discussion (Sutton et al, 1991). Noninvasive implants show little response to conventional chemotherapy. They seem to be indolent lesions that can be stable for a long time without therapeutic intervention. In contrast, invasive implants are treated like advanced ovarian carcinomas with peritoneal metastases.
The genesis and pathobiology of extraovarian lesions of SBOT remains enigmatic. Some investigators regard them as independent foci of serous epithelial proliferation, which are derived from the mesothelial cell layer that covers the ovarian surface and the pelvic peritoneum—the so-called secondary Müllerian system (Kadar and Krumerman, 1995). In contrast, others favor the notion that extraovarian foci of SBOT are the result of metastatic spread from the ovarian tumor (Moore et al, 2000). This theory may be suitable for ovarian borderline tumors that are located on the surface of the ovary but cannot easily explain extraovarian lesions associated with cystic ovarian borderline tumors that are clearly separated from the ovarian surface and peritoneal cavity (Segal and Hart, 1992).
So far only a limited number of molecular genetic alterations have been described in serous borderline tumors. Numerical chromosomal aberrations have been reported, but complex karyotypes are rare (Diebold et al, 1996). In contrast to invasive serous carcinomas that infrequently contain mutations of the K-RAS gene, alteration of this oncogene belongs to the most frequent aberrations in SBOT and can be detected in approximately one third of cases (Haas et al, 1999a; Mok et al, 1993; Taylor et al, 1995; Teneriello et al, 1993). K-RAS mutations mostly affect codon 12 of exon 1. In approximately 80%, GGT to GTT point mutations are found. This results in continuous activation of the Ras signaling cascade, leading to increased cell proliferation.
In the past, detailed analyses of extraovarian foci of SBOT were hampered by the small size of these lesions. By advances in laser-assisted microdissection, it was possible to overcome these obstacles. Using this technology, first studies of X-chromosome inactivation have provided data that show that at least some advanced borderline tumors are multifocal (Gu et al, 2001; Lu et al, 1998).
We hypothesized that analysis of the K-RAS mutation status might further help to clarify the relation of ovarian and extraovarian lesions of serous borderline tumors. Recently, (Alvarez et al 2001) reported on K-RAS mutations in Müllerian inclusion cysts associated with SBOT. In two of three cases, identical mutations were found. To get a more comprehensive view of the mutation status in advanced borderline tumors, we separately studied multiple samples of 40 microdissected extraovarian lesions and 8 contralateral tumors of 10 serous borderline tumors. Four of 10 borderline tumors had been part of a previous investigation in which we had analyzed the K-RAS mutation status in benign, borderline, and malignant serous tumors of the ovary (Haas et al, 1999a). In the present study, we provide evidence that bilateral and advanced borderline tumors with K-RAS mutation harbor identical mutations in all contralateral tumors and in a large number of extraovarian lesions.
Results
Eight cases of SBOT with known K-RAS mutation (RAS+) and two cases with wild-type K-RAS (RAS−) were analyzed for comparison. DNA extraction and K-RAS mutation analysis were successful in a total of 97 samples of 58 paraffin-embedded and laser-microdissected ovarian and extraovarian lesions (10 ovarian tumors, 8 ovarian tumors, 25 noninvasive implants, 15 Müllerian inclusion cysts without cytologic atypia). A minimum of 30 to 40 microdissected cells were necessary for the mutation analysis (Fig. 1). The localization of the samples and their morphology and the results of K-RAS analysis are listed in Table 1.
Example of K-RAS mutation analysis in a serous borderline tumor of the ovary (SBOT; case 304, sample 1). A RAS mutation was revealed first by denaturing gradient gel electrophoresis (DGGE; A) and then characterized as GGT → GTT mutation (Gly to Val) in two direct sequencing reactions (B and C). Marks point to the mutation in codon 12. The presence of the guanine base in tumor tissue is due to the second allele, which is not mutated. mut, mutation; wt, wild-type.
Five of eight RAS+ ovarian borderline tumors contained a GGT → GTT mutation (Gly to Val), two ovarian tumors had a GGT → GAT mutation (Gly to Asp), and one case had a GGT → GCT mutation (Gly to Ala) at codon 12 of the K-RAS gene.
To address the question of whether papillary serous epithelial lesions of borderline malignancy consist of polyclonal or monoclonal cell populations, we analyzed multiple samples from the same site in 16 ovarian and extraovarian lesions. Between two and five samples were studied per lesion. In 12 of 14 ovarian borderline tumors and in 2 of 2 extraovarian implants, identical K-RAS mutations could be found in different areas of the same lesion.
In seven of eight RAS+ cases, additional lesions could be analyzed. In six borderline tumors, tumor tissue was available from the second ovary. All contralateral tumors (six of six) harbored an identical mutation (Figs. 2 and 3). An example for the denaturing gradient gel electrophoresis pattern of a bilateral tumor (case 302) is given in Figure 4. In case 322 (Fig. 3), the bilateral cystic borderline tumor was characterized by a GGT → GCT mutation, and a separate surface borderline tumor component contained a GGT → GTT mutation in addition.
Bilateral SBOT (case 302); results of DGGE in correlation to histology. A K-RAS mutation is present in four of five samples. Sequencing revealed a GGT to GTT nucleic acid base change. Lane 1, normal control; lanes 2 and 3, surface borderline tumor of the left ovary; lanes 4 to 6, cystic borderline tumor of the right ovary.
Extraovarian lesions were available in five RAS+ cases. In 4 of these, RAS mutations were also found in implants (15/21 implants [71%]) and in Müllerian inclusion cysts (3/12 lesions [25%]). The extraovarian mutations were always identical to the one in the ovary (18 of 18 [100%]).
In case 322 with two different K-RAS mutations, the mutation of the bilateral cystic borderline tumor was also found in five implants located next to the uterus on both sides, whereas the mutation of the surface borderline tumor of the right ovary was also detected in four implants on the right uterine and parasalpingeal peritoneum and in two Müllerian inclusion cysts in right pelvic lymph nodes (Fig. 3). Two ovarian borderline tumors without K-RAS mutation (cases 323 and 324) were analyzed for comparison. In these cases, the contralateral tumors and seven extraovarian foci were available. In case 323, a single extraovarian implant contained a RAS mutation (glycine to valine).
Discussion
Molecular pathologic investigations have provided evidence that extraovarian lesions of advanced invasive ovarian carcinomas are derived from the primary tumor in the ovary. Analyzing the p53 gene, (Jacobs et al 1992) and (Mok et al 1992) found identical mutations at multiple sites of invasive carcinomas. (Cuatrecasas et al 1996) used the K-RAS gene to address this question in mucinous tumors of the ovary associated with pseudomyxoma peritonei. Demonstrating identical mutations at different localizations, they concluded that the ovarian tumors are closely related to synchronous appendiceal tumors.
Morphology and molecular alterations of SBOT strikingly differ from invasive ovarian carcinomas. p53 mutations, high-level loss of heterozygosity (LOH), and complex karyotypes are uncommon; in contrast, K-RAS alterations are found in approximately one third of serous borderline tumors (Diebold et al, 1996; Mok et al, 1993; Taylor et al, 1995; Teneriello et al, 1993). This indicates that the pathways that lead to the development of SBOT are different from those of carcinomas. It has been suggested that the extraovarian and contralateral lesions associated with SBOT also evolve in a different way. The clonal origin of these foci is still under discussion. Although some investigators think that they might represent metastases (Moore et al, 2000), others propose that a field effect promotes independent transformation of epithelial cells at different locations of the so-called secondary Müllerian system (Kadar and Krumerman, 1995; Lauchlan, 1990).
In the present study, we used K-RAS point mutations to compare a large number of laser-microdissected ovarian and extraovarian lesions of SBOT on a molecular level. In 16 lesions, we were able to analyze multiple samples from the same localization separately. The demonstration of identical mutations in the vast majority of cases clearly suggests that SBOT and their implants consist of monoclonal cell populations, which is in good agreement with the results of X-chromosome inactivation studies (Gu et al, 2001; Lu et al, 1998). Although it has been suggested that induction of Müllerian metaplasia may be an early step in the development of SBOT (Feeley and Wells, 2001), these data show that SBOT and associated implants do not represent reactive alterations of the secondary Müllerian epithelium but clearly are neoplastic lesions.
Recently, (Alvarez et al 2001) reported the results of a K-RAS analysis of three borderline tumors with Müllerian inclusion cysts. In two of three cases, identical mutations were found. In contrast to these authors, who used pooled DNA from multiple Müllerian inclusion cysts, we analyzed all lesions separately. In our study, 12 inclusion cysts of 5 patients with borderline tumors could be evaluated. In two cases (40%), a total of three cysts showed a RAS mutation. Although methodological problems cannot be totally excluded, it seems that even in cases with RAS+ Müllerian inclusion cysts, this mutation is not present in all of them.
Implants associated with serous SBOT have not been analyzed so far with regard to their K-RAS status. We saw K-RAS mutations in implants in four of five SBOT. Furthermore, all contralateral ovarian tumors also contained mutations. The demonstration of identical K-RAS mutations in these lesions suggests that they may be related like metastases of invasive carcinomas to their primary in the ovary. In this regard, it is noteworthy that (Moore et al 2000) found a statistically significant association of Müllerian inclusion cysts in lymph nodes with SBOT but not with other tumors of the female genital tract. They concluded that this might indicate a metastatic mechanism of development. However, a field effect acting on the pelvic peritoneum could conceivably lead to independent multifocal proliferation of Müllerian epithelium, too.
The identification of identical K-RAS mutations at different localizations is not completely incompatible with multifocality. The spectrum of K-RAS mutations in SBOT is small. Codon 12 point mutation is the most frequent one. It is found in approximately 80% of cases with K-RAS alteration (Haas et al, 1999a; Mok et al, 1993; Taylor et al, 1995; Teneriello et al, 1993). Thus, by chance, this mutation might develop independently in separate lesions. Supporting this notion, we could demonstrate a codon 12 glycine to valine mutation in an implant associated with an SBOT without RAS mutation.
Apparently, K-RAS mutations are early events in the development of extraovarian and ovarian serous epithelial proliferations. For comparison, in the pancreatic duct, epithelium K-RAS alterations have been described not only in carcinomas but also in benign lesions, including normal, hyperplastic, and metaplastic epithelium (Lüttges et al, 1999). In the colon, it has been suggested that some K-RAS mutations might be the result of epigenetic changes (Esteller et al, 2000). Thus, K-RAS mutations may represent a “field defect” caused by not-yet-known hormonal or environmental factors. Furthermore, it is conceivable that genetic predisposition such as that leading to familial colonic adenomas and carcinomas causes simultaneous tumorigenesis in ovarian and extraovarian tissues. However, epidemiology has not given any hints in this direction, and germline mutations have not been described.
Recent investigations of advanced SBOT that determined the X-chromosome inactivation pattern suggested multifocal development of bilateral tumors and extraovarian implants. (Lu et al 1998) found different patterns of X-chromosome inactivation in three of eight cases. However, in five cases, the inactivation pattern was identical at different sites, which can have happened by chance or may suggest a common origin. Using the same assay, (Gu et al 2001) found different inactivation patterns in six of seven tumors comparing implants and ovarian lesions and in three of seven bilateral ovarian tumors. Although these findings indicate multifocality, they do not rule out that that at least some lesions are derived from a common progenitor cell. In addition, the technique of X-chromosome inactivation analysis is applicable only to a minority of cases of SBOT.
The cases of the present study that demonstrated rarer types of codon 12 K-RAS mutations (glycine to asparagine and glycine to alanine) both in the ovarian tumor and in extraovarian lesions are difficult to explain by multifocality, in particular, the case with two different mutations that both were detected at several different locations in the ovaries and in pelvic tissue is puzzling. In this case, at least the lesions containing the glycine to alanine mutation seem to be related to the surface SBOT of the right ovary. This notion could be strengthened if one could compare other molecular markers of the lesions under study. Unfortunately, we do not know the X-chromosome inactivation pattern of our cases. The scarcity of the material made it impossible to perform additional analyses for which larger amounts of DNA are needed.
Regarding our observations, the designation of extraovarian lesions as “implants” may be justified in at least some cases. The determination of the clonal origin of contralateral and peritoneal lesions of SBOT may have important clinical implications. The prognosis of advanced SBOT is much more favorable than for invasive ovarian carcinomas. However, a 15-year mortality rate of 27% has been reported (Leake et al, 1992). Our data could be interpreted as indication that some patients might benefit from heightened surveillance and/or adjuvant therapy. However, at present, chemotherapy is reserved for women with invasive implants. It is not proved that adjuvant therapy has a positive effect in patients with noninvasive implants (Sutton et al, 1991). Apparently, because of their low proliferation rate, SBOT have an indolent course and respond poorly to chemotherapy. Therefore, even if some extraovarian lesions represent true metastases, adjuvant chemotherapy is not necessarily indicated.
The results of the present study and published data suggest that SBOT are a heterogenous group of tumors. K-RAS mutations are found only in one third of cases, and two thirds show (mostly low level) microsatellite instability (Haas et al, 1999b). Both phenomena may be related (Diebold, 2001) as has been described for colon carcinomas (Jass et al, 1999). Loss of heterozygosity is seen infrequently; complex genetic alterations and p53 mutations are detected only in a few cases (Diebold, 2001; Diebold et al, 1996; Haas et al, 1999b; Teneriello et al, 1993). The present study suggests that the pathways that lead to the development of extraovarian manifestations of the disease might also be variable. In SBOT with K-RAS mutation, a larger fraction of extraovarian foci could be due to metastatic spread, whereas in the remaining cases, these lesions could be mostly multifocal. However, as evidenced by one case in the present series, multifocal development and metastatic spread from the ovarian tumor might even occur at the same time. Apparently, these two possible pathways are not mutually exclusive. For completely resolving the question of the origin of extraovarian lesions in SBOT, a comparative study looking at multiple molecular changes simultaneously is probably needed.
Materials and Methods
Patient Samples
The cases were retrieved from the files of the Institute of Pathology of the University of Munich. All patients had undergone surgery between 1983 and 2000 at the Department of Gynecology and Obstetrics of the University Hospital at Groβhadern, Munich. A total of 10 patients were included in the study. Patient age ranged from 26 to 78 years (mean 45.3). Tissue sources were the reproductive tract itself as well as pelvic peritoneum, pelvic lymph nodes, omentum, large bowel, and small bowel. The tissue was routinely formalin-fixed and paraffin-embedded. Pathomorphological assessment was performed after routine hematoxylin/eosin staining of 4-μm tissue sections. Using the criteria of the International Federation of Gynecology and Obstetrics, three cases (123, 302, 321) were stage I, three cases (320, 322, 324) were stage II, and four cases (107, 127, 304, 323) were stage III.
Laser Microdissection and DNA Extraction
Paraffin blocks bearing ovarian borderline tumor tissue or extraovarian lesions were sectioned at 2-μm thickness, mounted on microscope slides previously covered with a 1,35-μm PEN membrane (P.A.L.M. Mikrolaser Technology, Bernried, Germany), and dried at 55°C. Sections were stained with methylene blue. Microdissection was carried out by means of a laser (P.A.L.M. Mikrolaser Technology). Dissected tissue was transferred directly into 25 μl of lysis buffer (50 mm Tris-HCl [pH 8.5]/1 mm EDTA [pH 8.0]/0.5% Tween-20/200 pg/μl proteinase K) and incubated at 55°C overnight.
Proteinase K was heat-inactivated by a 10-minute incubation at 95°C. Subsequently, tissue debris was pelleted by centrifugation at 14,000 rpm for 5 minutes, and the supernatant was transferred to a fresh Eppendorf tube. DNA was extracted by using the EZNA Tissue DNA extraction Kit II (Peqlab, Erlangen, Germany). Microdissection of 100 to 200 cells yielded approximately 1 ng of DNA.
K-RAS Analysis
For denaturing gradient gel electrophoresis, exon 1 of K-RAS was amplified by PCR in a reaction mixture containing 10 mm Tris-HCl (pH 8.3), 50 mm KCl, 2 mm MgCl2, 200 μm of each deoxynucleotide trisphosphate (Amersham Pharmacia, Uppsala, Sweden), 0.2 pmol/μl of each primer, 0.025 U/μl AmpliTaq Gold (Applied Biosystems, Foster City, California), and 0.2 ng of template. The primer sequences have been described (Imai et al, 1994). PCR conditions were as follows: 10 minutes at 94°C followed by 40 to 45 cycles of 94°C for 2 minutes, 52°C for 2 minutes, and 72°C for 2 minutes. After the final cycle, another 8 minutes at 72°C was added. The PCR products were then run on a 10% polyacrylamide gel with a vertical denaturing gradient from 20 to 60% (100% denaturant corresponds to 7 M urea and 40% formamide). Electrophoresis was performed on a Dcode System (BioRad, Hercules, California) at 60°C with constant voltage (150 V) for 6 hours. Finally, the gel was stained with ethidium bromide and evaluated on an ultraviolet screen.
For sequencing, the DNA was amplified under the same PCR conditions as described by (Sarkar et al 1995). The PCR products were purified with a PCR purification kit (Quiagen, Hilden, Germany). After cycle sequencing with a dye terminator cycle sequencing kit (Applied Biosystems) according to the manufacturer's instructions, the products were purified with centri-sep columns (Princeton Separations, Adelphia, New Jersey). The sequences were analyzed on an ABI PRISM 310 genetic analyzer (Applied Biosystems). For each sample, forward and reverse sequences were determined. In all samples that contained K-RAS mutations, the results were reproduced at least two to three times in repeated analyses.
References
Alvarez AA, Moore WF, Robboy SJ, Bentley RC, Gumbs C, Futreal PA, and Berchuck A (2001). K-ras mutations in Müllerian inclusion cysts associated with serous borderline tumors of the ovary. Gynecol Oncol 80: 201–206.
Chambers JT, Merino MJ, Kohorn EI, and Schwartz PE (1988). Borderline ovarian tumors. Am J Obstet Gynecol 37: 1088–1094.
Cuatrecasas M, Matias-Guiu X, and Prat J (1996). Synchronous mucinous tumors of the appendix and the ovary associated with pseudomyxoma peritonei. A clinicopathologic study of six cases with comparative analysis of c-Ki-ras mutations. Am J Surg Pathol 20: 739–746.
Diebold J (2001). Phenotype–genotype correlation in ovarian neoplasia. Verh Dtsch Ges Pathol 85: 153–160.
Diebold J, Deisenhofer I, Baretton GB, Blasenbreu S, Suchy B, Schneiderbanger K, Meier W, Haas CJ, and Löhrs U (1996). Interphase cytogenetic analysis of serous ovarian tumors of low malignant potential: Comparison with serous cystadenomas and invasive serous carcinomas. Lab Invest 75: 473–485.
Esteller M, Toyota M, Sanchez-Cespedes M, Capella G, Peinado MA, Watkins DN, Issa JP, Sidransky D, Baylin SB, and Herman JG (2000). Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is associated with G to A mutations in K-ras in colorectal tumorigenesis. Cancer Res 60: 2368–2371.
Feeley KM and Wells M (2001). Precursor lesions of ovarian epithelial malignancy. Histopathology 38: 87–95.
Gu J, Roth LM, Younger C, Michael H, Abdul-Karim FW, Zhang S, Ulbright TM, Eble JN, and Cheng L (2001). Molecular evidence for the independent origin of extra-ovarian papillary serous tumors of low malignant potential. J Natl Cancer Inst 93: 1147–1152.
Haas CJ, Diebold J, Hirschmann A, Rohrbach H, and Löhrs U (1999a). In serous ovarian neoplasms the frequency of Ki-ras mutations correlates with their malignant potential. Virchows Arch 434: 117–120.
Haas CJ, Diebold J, Hirschmann A, Rohrbach H, Schmid S, and Löhrs U (1999b). Microsatellite analysis in serous tumors of the ovary. Int J Gynecol Pathol 18: 158–162.
Imai M, Hoshi T, and Ogawa K (1994). K-ras codon 12 mutations in biliary tract tumours detected by polymerase chain reaction denaturing gradient gel electrophoresis. Cancer 73: 2727–2733.
Jacobs IJ, Kohler MF, Wiseman RW, Mars JR, Whitaker R, Kerns BA, Humphrey P, Berchuck A, Ponder BA, and Bast RC (1992). Clonal origin of epithelial ovarian carcinoma: Analysis by loss of heterozygosity, p53 mutation, and X-chromosome inactivation. J Natl Cancer Inst 84: 1793–1798.
Jass JR, Biden KG, Cummings MC, Simms LA, Walsh M, Schoch E, Meltzer SJ, Wright C, Searle J, Young J, and Leggett BA (1999). Characterization of a subtype of colorectal cancer combining features of the suppressor and mild mutator pathways. J Clin Pathol 52: 455–460.
Kadar N and Krumerman M (1995). Possible metaplastic origin of lymph node “metastases” in serous ovarian tumor of low malignant potential (borderline serous tumor). Gynecol Oncol 59: 394–397.
Kennedy AW and Hart WR (1996). Ovarian papillary serous tumors of low malignant potential (serous borderline tumors): A long-term follow-up study, including patients with microinvasion, lymph node metastasis, and transformation to invasive serous carcinoma. Cancer 78: 278–286.
Kurman RJ and Trimble CL (1993). The behaviour of serous tumors of low malignant potential: Are they ever malignant? Int J Gynecol Pathol 12: 120–127.
Lauchlan SC (1990). Non-invasive ovarian carcinoma. Int J Gynecol Pathol 9: 158–169.
Leake JF, Currie JL, Rosenshein NB, and Woodruff JD (1992). Long-term follow-up of serous ovarian tumors of low malignant potential. Gynecol Oncol 47: 150–158.
Lu KH, Bell DA, Welch WR, Berkowitz RS, and Mok SC (1998). Evidence for the multifocal origin of bilateral and advanced human serous borderline ovarian tumors. Cancer Res 58: 2328–2330.
Lüttges J, Schlehe B, Menke MA, Vogel I, Henne-Bruns D, and Klöel G (1999). The K-ras mutation pattern in pancreatic ductal adenocarcinoma usually is identical to that in associated normal, hyperplastic, and metaplastic ductal epithelium. Cancer 85: 1703–1710.
Mok SH, Bell DA, Knapp RC, Fishbaugh PM, Welch WR, Muto MG, Berkowitz RS, and Tsao SW (1993). Mutation of K-ras protooncogene in human ovarian epithelial tumors of borderline malignancy. Cancer Res 53: 1489–1492.
Mok SH, Tsao SW, Knapp RC, Fishbaugh PM, and Lau CC (1992). Unifocal origin of advanced human epithelial ovarian carcinomas. Cancer Res 52: 5119–5122.
Moore WF, Bentley RC, Berchuck, and Robboy SJ (2000). Some Müllerian inclusion cysts in lymph nodes may sometimes be metastasis from serous borderline tumors of the ovary. Am J Surg Pathol 24: 710–718.
Sarkar FH, Valdivieso M, Borders J, Yao KL, Raval MMT, Madan SK, Sreepathi P, Shimoyama R, Steiger Z, Visscher DW, and Crissman JD (1995). A universal method for the mutational analysis of K-ras and p53 gene in non-small cell lung cancer using formalin-fixed paraffin-embedded tissue. Diagn Mol Pathol 4: 266–273.
Segal GH, and Hart WR (1992). Ovarian serous tumors of low malignant potential (serous borderline tumors). The relationship of exophytic surface tumor to peritoneal “implants.” Am J Surg Pathol 16: 577–583.
Sutton GP, Bundy BN, Omura GA, Yordan EL, Beecham JB, and Bonfiglio T (1991). Stage III ovarian tumors of low malignant potential treated with cisplatin combination therapy (a Gynecologic Oncology Group study). Gynecol Oncol 41: 230–233.
Taylor RR, Linnoila RI, Gerardts J, Teneriello MG, Nash JD, Park RC, and Birrer MJ (1995). Abnormal expression of the retinoblastoma gene in ovarian neoplasms and correlation to p53 and K-ras mutations. Gynecol Oncol 58: 307–311.
Teneriello MG, Ebina M, Linnoila RI, Henry M, Nash JD, Park RC, and Birrer MJ (1993). p53 and Ki-ras gene mutations in epithelial ovarian neoplasms. Cancer Res 53: 3103–3108.
Acknowledgements
We thank Christian Haas and Astrid Hirschmann for comments and expert technical assistance.
This work was supported by grant DI 774/2-1 (to JD) from the Deutsche Forschungsgemeinschaft.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Diebold, J., Seemüller, F. & Löhrs, U. K-RAS Mutations in Ovarian and Extraovarian Lesions of Serous Tumors of Borderline Malignancy. Lab Invest 83, 251–258 (2003). https://doi.org/10.1097/01.LAB.0000056994.81259.32
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1097/01.LAB.0000056994.81259.32
This article is cited by
-
Seröse Tumoren des Ovars
Der Pathologe (2014)
-
Frequency of mutations and polymorphisms in borderline ovarian tumors of known cancer genes
Modern Pathology (2013)
-
Molecular Characterization of 103 Ovarian Serous and Mucinous Tumors
Pathology & Oncology Research (2011)
-
KRAS mutation analysis in ovarian samples using a high sensitivity biochip assay
BMC Cancer (2009)
-
Borderline-Tumor des Ovars
Der Gynäkologe (2006)