Abstract
Study design:
Randomized study.
Objectives:
To evaluate the effects of thalidomide on spinal cord ischemia/reperfusion injury via reduced TNF-α production.
Setting:
Animal experimental laboratory, Clinical Research Institute of Seoul National University Hospital, Seoul, Korea.
Methods:
Spinal cord ischemia was induced in rabbits by occluding the infrarenal aorta. Rabbits in group N did not undergo ischemic insult, but rabbits in groups C (the untreated group), THA, and THB underwent ischemic insult for 15 min. The THA and THB groups received thalidomide (20 mg/kg) intraperitoneally (i.p.) before ischemia, but only the THB group received thalidomide (i.p., 20 mg/kg) after 24 and 48 h of reperfusion. After evaluating neurologic functions at 1.5 h, 3, and 5 days of reperfusion, rabbits were killed for histopathologic examination and Western blot analysis of TNF-α.
Results:
The THA and THB groups showed significantly less neurologic dysfunction than the C group at 1.5 h, 3, and 5 days of reperfusion. The number of normal spinal motor neurons in ventral gray matter was higher in THA and THB than in C, but no difference was observed between THA and THB. Western blot analysis showed a significantly higher level of TNF-α in C than in THA and THB at 1.5 h of reperfusion, but no difference was observed between C, THA, or THB at 3 or 5 days of reperfusion.
Conclusion:
Thalidomide treatment before ischemic insult reduces early phase ischemia/reperfusion injury of the spinal cord in rabbits.
Similar content being viewed by others
Introduction
Mortality rates due to intra-operative events have diminished remarkably over the past 30 years in the surgical management of patients with diseases of the descending thoracic aorta and thoracoabdominal aorta. However, postoperative spinal cord injuries remain serious complications, and the prevalence of paraplegia for this complication type has been reported to be between 0.9 and 40%.1 Moreover, it is presumed that the main mechanism of spinal cord injury involves ischemia/reperfusion injury, however, the details have been unknown.
Immunoreactivity for TNF-α was observed in the white matter of the mouse hippocampus as early as 1.5 h after 30 min of global ischemic insult. This then decreased at 6 and 24 h, only to reincrease at 3 days.2 Moreover, focal cerebral ischemia in rats results in elevated TNF-α mRNA and protein in ischemic neurons.3 In human victims of fatal ischemic stroke, cells throughout the brain show TNF-α immunoreactivity during the different phases of focal cerebral infarction with no or incomplete recanalization.4 Moreover, reduced systemic TNF-α release was associated with protection from spinal cord reperfusion injury in rabbits.5
Thalidomide, a derivative of glutamic acid, was available as an over-the-counter drug in Europe during the late 1950s for the treatment of morning sickness, but was withdrawn in the 1960s after the emergence of reports of teratogenicity and phocomelia associated with its use. The recent return of thalidomide stems from its broad-spectrum pharmacologic and immunologic effects. Thalidomide has been approved by the Food and Drug Administration for the treatment of erythema nodosum leprosum and an inflammatory manifestations of leprosy, and its potential therapeutic applications span a wide spectrum of other diseases.6 It has been reported that the mode of action of thalidomide involves the inhibition of lipopolysaccharide (LPS)-induced TNF-α production, and the enhanced degradation of TNF-α mRNA.7
Given the background described above, the hypothesis of this study was that thalidomide is likely to reduce spinal cord ischemia/reperfusion injury, and that this protection is due to a reduction in the quantity of TNF-α expressed in the spinal cord. To evaluate this hypothesis, we used an ischemia/reperfusion rabbit model.
Materials and methods
Animal model
In this experimental study, rabbits were treated in accordance with the NIH Guide for the Care and Use of Animals. All procedures and animal care protocols were approved by the Animal Care Committee of the Clinical Research Institute of Seoul National University Hospital.
The present study used 54 male New Zealand White Rabbits of body weight 2.5–3 kg. Ketamine hydrochloride (50 mg/kg, Yuhan Yanghang, Seoul) and xylazine hydrochloride (5 mg/kg, Bayer Korea, Seoul) were injected intramuscularly to induce anesthesia; endotracheal intubation and tracheostomy were not performed.
Rabbits were supplied oxygen at 6 l/min via a facemask, while spontaneous respiration was maintained. A 24 G catheter (D&B-cath®, Boin Medica, Kumi, Korea) of length 0.75 inch was located in the auricular vein, and xylazine hydrochloride was intravenously infused at 2.5 mg/kg, equivalent to a half of the anesthetic induction dose, to maintain anesthesia.
After EKG, pulse oximetry and rectal temperature were monitored, and a 24 G catheter (D&B-cath®, Boin Medica, Kumi, Korea) of length 0.75 inch was located in the auricular artery to monitor blood pressure.
After intravenously injecting cefazolin sodium (Chong Kun Dang, Seoul) at 10 mg/kg, bilateral femoral arteriotomy was performed and a 22 G catheter (D&B-cath®, Boin Medica, Kumi, Korea) of length 0.75 inch was located in the right femoral artery to monitor blood pressure and blood pressure waves distal to the future aorta occlusion. After intravenously injecting heparin (Choongwae, Seoul) at a dose of 150 IU/kg, a 5-Fr pulmonary artery catheter (Balloon thermodilution catheter®, Arrow, Reading, PA, USA) was inserted 3–4 cm caudally to the inguinal ligament into the left femoral artery, and then advanced 15 cm further into the abdominal aorta. Thus, the balloon at the distal end of the catheter was positioned 0.5–1.5 cm distally to the origin of the left renal artery. Other study8 and preliminary investigations by injecting radiocontrast dye into the abdominal aorta via the distal hole of the catheter or by laparotomy confirmed that the balloon was positioned 0.5–1.5 cm distal to the left renal artery (Figure 1). PaO2, PaCO2 and Hb were performed and pH was measured at baseline, during ischemia, and 10 min of reperfusion following ischemia. To maintain body temperature within the normal range perioperatively, an automated circulating water blanket was used throughout. While inducing ischemia, we monitored blood pressure waves at the proximal and distal holes of the pulmonary catheter and compared these with those of the auricular artery to prevent twisting or contralateral insertion of the catheter. After the procedure, the all catheters were removed and 0.5% bupivacaine was infiltrated around the incision sites to minimize pain. Animals were allowed to recover from anesthesia at room temperature for at least 1.5 h, at which time it was possible to establish whether the motor activities of hind-limbs were normal. They were then returned to their cage and allowed free access to food and water.
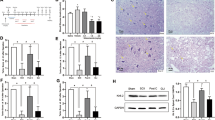
This photograph shows that the tip of a pulmonary artery catheter with an inflated balloon located below the left renal artery. The pulmonary artery catheter was inserted 3–4 cm caudally to the inguinal ligament into the femoral artery, and then advanced 15 cm further into the abdominal aorta. Thus, the balloon at the distal end of the catheter is positioned 0.5–1.5 cm distally to the origin of the left renal artery
Experiment protocol
In Group N (n=6) animals, the balloon-tipped catheter was placed in the abdominal aorta but not inflated. In the Groups C (n=18), THA (n=18) and THB (n=12), the catheter balloon was inflated for 15 min so as to cause spinal cord ischemia.8 Rabbits in Groups THA and THB received thalidomide (20 mg/kg, Sigma-Aldrich Corporation, St Louis, MO) with vehicle (dimethyl sulfoxide, DMSO, Sigma-Aldrich Corporation, St Louis, MO) i.p. 3 h before the initiation of aortic occlusion. Rabbits in Group C received only vehicle without thalidomide i.p. 3 h before aortic occlusion. Rabbits in Group THB also received thalidomide (20 mg/kg; i.p.) with vehicle at 24 and 48 h of reperfusion, whereas rabbits in Groups C and THA only received vehicle at this time. Six animals from the N (N), C (C-1), and THA (THA-1) were euthanized with a high dose of thiopental sodium (50 mg/kg, Choongwae, Seoul) injected intravenously after intramuscular ketamine hydrochloride (25 mg/kg) and xylazine hydrochloride (5 mg/kg) at 1.5 h of reperfusion, and their spinal cords were quickly removed. Similarly, six animals from the C (C-3), THA (THA-3), and THB (THB-3) groups and six animals from the C (C-5), THA (THA-5), and THB (THB-5) groups were euthanized at 3 and 5 days, respectively, of reperfusion and spinal cords were removed as described above. Tissue samples for Western blot analysis (spinal cords at the L7 level) were frozen in liquid nitrogen and then stored at −80°C. Samples for histology, taken at the L5–L6 level, were fixed by immersion in 0.1 mol/l phosphate buffer containing 10% paraformaldehyde for 2 weeks and then embedded in paraffin.
Neurologic assessment
Hind-limb motor function was observed at 1.5 h, at 3 days and at 5 days of reperfusion. Rabbits were classified using a 5-point scale according to the modified Tarlov scale9 (grade 0=no movement; 1=slight movement or minimal antigravity activity; 2=sits with assistance or active antigravity activity; 3=sits alone; 4=weak hop; 5=normal hop). Two investigators without knowledge of treatment histories independently graded neurologic scores.
Histopathologic examination
From each embedded spinal cord sample, three transverse tissue sections were prepared at 4 μm. These sections were stained with hematoxylin–eosin and examined by light microscopy. An investigator, unaware of group identities or neurologic outcomes, examined each slide under a light microscope and counted the number of normal nerve cells in the ventral horn of spinal cord gray matter (anterior to an imaginary line drawn through the central canal perpendicular to the vertical axis). In each rabbit, mean normal nerve cell number was calculated from mean numbers in the three spinal cord tissue sections. This value was used as the representative number in each rabbit.
Western blot analysis
TNF-α contents were semiquantitatively determined by Western blotting and chemiluminescence. To determine TNF-α levels, frozen spinal cords were individually homogenized using lysis buffer (pH 7.4 50 mM Tris, 1% Na-deoxychloride, 0.5% SDS, 0.1% DW, 25 mM Triton X-100) and protein levels were estimated using the Bradford method. Samples (30 μg) of protein from each rabbit were electrophoresed in 12% sodium dodecyl sulfate-polyacrylamide gel and transferred to nitrocellulose membranes, which were then blocked in 2.5% powdered milk dissolved in TBS+0.05% Tween 20 for 10 min at room temperature. Membranes were then rinsed in TBS+0.05% Tween 20 for 3 × 15 min. The primary antibody, anti-TNF-α monoclonal Ab (Sigma-Aldrich Corporation, St Louis, MO) was diluted 1:1000 in TBS and incubated overnight at 4°C. The following day, membranes were rinsed for 3 × 15 min in TBS+0.05% Tween 20, and then the secondary antibody, horseradish peroxidase (HRP)-conjugated anti-mouse IgG (Santa Cruz Biotechnology, Santa Cruz, CA) was administered (diluted in 1:2000 in TBS) and incubated for 1 h at room temperature. Membranes were finally rinsed in TBS+0.05% Tween 20 for 3 × 15 min, and the antigen–antibody complex was visualized using an enhanced chemiluminescence Western blotting detection kit (Amersham, Arlington Heights, IL). Data were quantified by densitometry (Labworks Software, Upland, CA). Recombinant pure TNF-α (Endogen®, Pierce Biotechnology, Rockford, IL) was used as positive control (25 pg) and data are expressed as percentage of the control.
Statistical analysis
All numerical data are presented as means±s.d. Neurologic scores and neuron counts were analyzed via the Kruskal–Wallis procedure followed by the Mann–Whitney U-test. Overall group differences of the physiological variables and TNF-α contents were compared by analysis of variance (ANOVA), followed by the Tukey procedure for multiple comparisons when ANOVA showed a statistical significance. Statistical analysis was performed using SAS (The SAS System®, SAS Institute, Cary, NC). Statistical significance was set at P<0.05.
Results
Rabbits' hemodynamic profiles and temperatures are represented in Table 1. In Groups C, THA, and THB, blood pressure in the femoral artery reduced to almost zero with no pulsation after the catheter balloon was inflated, whereas that of the auricular artery was elevated and 2–3 min later reduced to the prior level. Moreover, after deflating the catheter, the blood pressure of the femoral artery gradually reached the baseline level, and that of the auricular artery was transiently lowered and thereafter elevated to the prior level. In terms of hemodynamic profiles, Group N showed a significantly higher mean femoral arterial pressure than C, THA, and THB during the ischemic insult (P<0.05). However, no significant difference was noted between Group C, THA, and THB at this time.
The recorded body temperatures of all rabbits remained in the normal range throughout the surgical procedure.
In terms of results of PaO2, PaCO2, Hb and pH, no significant differences were observed between the groups.
Neurologic outcome
Neurologic results are summarized in Table 2. In Group N, all six rabbits were of modified Tarlov scale 5 at 1.5 h of reperfusion. At 1.5 h of reperfusion, the modified Tarlov scales of groups THA-1, THA-3, THA-5, THB-3, and THB-5 were significantly higher than those of Groups C-1, C-3 and C-5 (P<0.05). The modified Tarlov scales of Groups THA-3, THA-5, THB-3 and THB-5 were significantly higher than those of Groups C-3 and C-5 at 3 days of reperfusion (P<0.05), but no significant difference was found between the THA (THA-3, THA-5) and THB (THB-3, THB-5) groups. At 5 days of reperfusion, the modified Tarlov scales of the THA-5 and THB-5 subgroups were significantly higher than that of C-5 (P<0.05), but no significant difference was observed between THA-5 and THB-5.
Histopathology
Table 3 lists the number of viable normal motor nerve cells in the ventral horn of the spinal cord gray matter stained by hematoxylin–eosin. At 1.5 h of reperfusion, the number of normal motor nerve cells in Group N was higher than in Groups C or THA (P<0.05) and that in Group THA was higher than in Group C (P<0.05). At 3 days of reperfusion, the number of normal motor nerve cells in THA and THB were higher than in C (P<0.05), and there was no significant difference between THA and THB. At 5 days of reperfusion, THA and THB contained more normal motor nerve cells than C (P<0.05), but no significant difference was observed between THA and THB. Modified Tarlov scale grades were well correlated with the number of normal motor nerve cells in ventral gray matter.
Representative photomicrographs of sections stained with hematoxylin–eosin are shown in Figure 2. No notable features were found in motor neurons in Group N; however, in Group C-1 pyknotic nuclei, structureless cytoplasm, vacuolization and inflammatory reaction accompanying lymphocyte infiltration indicative of necrosis were noted. Shrunken motor nerve cells, loss of nuclei, and Nissl bodies were noted in Group C-5 at 5 days of reperfusion. On the other hand, no animal showed any change in the histopathological characteristics of sensory nerve cells.
Representative photomicrographs of spinal cord sections stained with hematoxylin–eosin 1.5 h (a–c), 3 days (d–f), and 5 days (g–i) after reperfusion. No neuronal damage was observed to any motor neuron cells in Group N (a), whereas the number of motor neuron cells in Group C-1 decreased significantly (b). Viable motor neuron cells were markedly more preserved in Group THA-1 (c), a–c; original magnification, × 40). Section from a rabbit with paraplegia in Group C-3, showing prominent vacuolization, lymphocytosis, and abnormal motor neuron cells with unclear nucleus (d; original magnification, × 400). Many motor neuron cells were well preserved in Group THA-3 (e; original magnification, × 400), and many normal motor neuron cells (arrow) containing nucleus and Nissl body were observed in Group THB-3 (f; original magnification, × 100). Motor neuron cells were shrunken and nucleoli were unclear (arrow) in Group C-5 (g). Normal motor neuron cells were observed in Groups THA-5 (H) and THB-5 (i), g–i; original magnification, × 200)
Western blot analysis
A characteristic accumulation of immunoreactive TNF-α was evident as a single band of molecular mass 17 kDa. Figure 3 shows representative Western blots and densitometry results for TNF-α expression in tissues from an ischemic spinal cord injury zone. TNF-α content was elevated at 3 days of reperfusion in the C (C-3, 92.4±5.7), THA (THA-3, 78.3±15.4), and THB (THB-3, 81.7±12.5) groups. At 1.5 h of reperfusion TNF-α levels in the N (23.0±5.1) and THA-1 (30.2±15.7) groups were lower than in the C-1 (53.4±12.3) group (P<0.05), but no significant difference was observed between these groups at 3 and 5 days (C-5, 40.4±20.1; THA-5, 36.1±12.9; THB-5, 33.2±14.1) of reperfusion.
Representative Western blots and densitometry values of TNF-α expression in tissues taken from ischemic zones. Group N: no ischemic insult, Group C: only ischemic insult, Group THA: preischemic thalidomide+ischemic insult, Group THB: preischemic thalidomide+ischemic insult+postischemic thalidomide, −1: sacrificed at 1.5 h after reperfusion, −3: sacrificed at 3 days after reperfusion, −5: sacrificed at 5 days after reperfusion. *P<0.05 versus Group N, THA-1
Discussion
Ischemia/reperfusion injury of central nervous system (CNS) is a complex, multistage process. It is initiated by the immediate damage caused by hypoxia as well as by return of oxygenated blood.10 The so-called secondary damage involves neuronal injury from excitatory amino acids, intracellular Ca2+ accumulation, free radical generation, and apoptosis as well as microvascular processes. There is accumulating evidence that inflammatory-immunologic reactions are also involved in the pathogenesis of CNS ischemia.11 Characterizing and ultimately controlling medication of cytokine inflammatory response may restrain its relative influence on ischemia/reperfusion injury.12
The present study provides first evidence that the intraperitoneal (i.p.) administration of thalidomide reduces ischemia/reperfusion injury in a rabbit spinal cord model via reduction of TNF-α expression.
TNF-α is a pleiotropic cytokine,13 but its role in ischemic spinal cord injury has not been fully elucidated. In ischemia/reperfusion injury, an excessive production of TNF-α may lead to activation of neutrophils and upregulation of cell adhesion molecules (CAMs) on both neutrophils and endothelial cells.14 CAMs cause leukocyte adherence to the endothelium, followed by transmigration into the interstitium, release of toxic enzymes, and tissue damage.15, 16 TNF-α has been shown to be associated with multiple organ and tissue ischemia/reperfusion injury.17, 18, 19 Increased production of TNF-α during permanent and transient ischemia in experimental models and clinical studies is well documented.3, 20 TNF-α induces cFOS and strongly potentiates glutamate-mediated cell death in the rat spinal cord.21 Its inhibition by pharmacological agents, neutralizing Abs, or soluble receptors has a protective effect.22
Although thalidomide and its analogues have broad spectrum properties that range from anti-inflammatory23 to antitumoral activities,6 its major mode of action is the inhibition of TNF-α synthesis.7, 24, 25 Thalidomide could modulate TNF-α production by several mechanisms, for example, by enhancing TNF-α mRNA degradation7 or by binding to α1-acid glycoprotein.26 Using real-time PCR, Kim et al27 confirmed that thalidomide lowers TNF-α level by enhancing the degradation of TNF-α mRNA. There is conflicting evidence about whether inhibition of NF-κB (a multisubunit trascription factor, whose activation can rapidly increase transcrition of various inflammatory cytokines and adhesion molecules28) activation account for the effects of thalidomide. Several lines of evidence suggest that thalidomide can inhibit DNA binding of NF-κB29, 30 but other studies have suggested that thalidomide exerts its immunomodulatory action by an NF-κB-independent mechanism.31, 32 The effect of thalidomide on TNF-α seemed to be rather specific, as Kim et al27 observed no change in IL-1β or IL-8 mRNA transcription. This finding concurs with a study that thalidomide inhibits only TNF-α and IL-12, not IL-1β and IL-6.33
In the present study, TNF-α expression in Group THA-1 was significantly reduced as compared with Group C-1, which is in line with the observation of high numbers of normal motor nerve cells in the spinal cords of Group THA-1. These results indicate that treatment with thalidomide before ischemic insult reduces early phase ischemia/reperfusion injury of the spinal cord.
However, TNF-α has neuroprotective effects, for example, it modulates essential mediators of cell protection,34 and it stimulates the expression of nerve growth factor in a variety of cell types including fibroblast and astrocytes, which may contribute to neuronal survival in and around an ischemic site.35 TNF-α induces manganese superoxide dismutase, which may protect against ischemia/reperfusion nerve injury by reducing reactive oxygen intermediate (ROI) in a variety of cells including neurons.36 These findings suggest that the function of TNF-α is target cell-dependent in the pathophysiology of brain and spinal cord injuries. In the present study, the expression of TNF-α was lower in Group THA than in Group C, and the number of normal motor nerve cells was higher in Group THA than Group C at 1.5 h of reperfusion. This suggests that expressed TNF-α at 1.5 h of reperfusion is neurotoxic. However, no further neurological damage or reductions in normal motor nerve cell numbers were observed at 3 and 5 days of reperfusion versus 1.5 h in any group, although TNF-α expression at 3 days in all groups was higher than at 1.5 h. These findings suggest that TNF-α at 3 and 5 days is either neuroprotective or unrelated to nerve cell pathophysiology.
These suggestions are explained by the following studies. TNF-α is a potent activator of necrosis and/or apoptosis through TNF Rp55 receptor depending on the cell type and/or the intracellular ATP concentration.37 TNF-α induces a wide spectrum of biological responses by interacting with two cell surface receptors, TNF receptor 1 (TNFR1) and 2 (TNFR2).38 Recent studies have shown that TNFR activation is not necessarily deleterious. TNF-α and TNFR may prevent neuronal apoptosis induced by excitotoxin in vitro39 and in vivo,40 cerebral ischemia,41 and traumatic brain injury.42 An important intermediate step in the TNFR-mediated signaling process is NF-κB activation43 and Marchetti et al44 proposed that TNF functions as an important regulatory cytokine in the central nervous system with differential signaling through the two distinct TNFRs determining its contribution to degenerative and regenerative processes. Thus, depending on the cellular composition of the affected tissue and intracellular availability of TNFR signaling components, TNF may function to aggravate or ameliorate disease.
Uno et al2 revealed the time course and the cellular sources of TNF-α in the brain following transient global ischemia in rats. The production of TNF-α peaked at 1.5 h and was reducing at 6 h, but reincreased 3 days after ischemic insult. The first peak was TNF-α attributed to microglia and the second to astrocytes. Similar TNF-α response profiles have been reported in cultured microglia and astrocytes stimulated with lipopolysaccharide,45, 46 in traumatized brains,47 and in the ischemic brain.3 The magnitude of cellular TNF response to cerebral ischemia is animal strain dependent in the mouse, whereas the time-profile and the cellular sources of TNF are similar regardless of genetic background.12 Furthermore, the lack of a correlation between infarct size and cellular TNF response suggests that the functionally important TNF is produced during the earliest phase (minutes to a few hours) by microglial cells after the induction of ischemia.12 Other findings support the above suggestion that microglia respond quickly after transient spinal cord ischemia in rabbits48 and that microglial activation precedes the manifestation of brain injury in mice.49
In the present study, TNF-α expression in Group C at 1.5 h and 3 days of reperfusion was higher than at 5 days, and at 3 days was higher than at 1.5 h. This time-profile is concordant with those found by other studies. In the thalidomide-treated groups THA and THB, TNF-α expression was highest at 3 days of reperfusion but that at 1.5 h was not higher than at 5 days, and was similar to that at 1.5 h in Group N. It is thought that this was due to the effect of thalidomide administered before ischemic insult. However, the administration of thalidomide at 24 and 48 h of reperfusion did not reduce the second TNF-α expressional peak. This suggests that the effect of thalidomide is target cell-dependent and that its target cells are microglia. This hypothesis is supported by the finding that thalidomide inhibits TNF-α production by lipopolysaccharide- or lipoarabinomannan-stimulated human microglial cells.50
Unfortunately, we did not assess the mechanism of the target cell-dependent effect of thalidomide. However, we propose that thalidomide may fuction as a inhibitor of TNF-α synthesis depending intracellular availability of transcription factors and α1-acid glycoprotein.
We chose 15 min of ischemia to allow comparison between our results and those of others.8, 51 However, no rabbit in the present study showed delayed motor neuron dysfunction or delayed motor neuron death. It is possible that the DMSO administered as vehicle, reduced delayed motor neuron dysfunction, death, and apoptosis, as the scavenging effects of DMSO, an antioxidative agent are known to attenuate apoptosis after ischemia/reperfusion.52 DMSO blocks the inactivation of mitochondrial aconitase, ATP depletion in astrocytes, and neuronal damage,53 and increases hypoxic tolerance in the brain.54
Kiyoshima et al55 reported that histopathologic and biochemical examinations showed no evidence of motor neuron apoptosis, but did show characteristic necrotic neuronal death in the rabbit spinal cord ischemia model after 15 min of ischemia, which is substantially in agreement with the findings of our study.
The doses of thalidomide administered in other studies25, 56, 57, 58 were decided upon without explanation. In the present study, we considered that the maximum recommended oral dose for the treatment of TB, AIDS, and leprosy in adult humans is 200–1200 mg/day, and by assuming an average body weight of 60 kg, we decided on a dose of 3–20 mg/kg/day. Thus, we determined the experimental dose of 20 mg/kg of thalidomide, which is a maximum for humans but is less than that used in other animal experiments. No side effects associated with thalidomide were observed in the present study. Thalidomide was reported to inhibit TNF-α production in a concentration-dependent manner in Langerhans cells.59 However, no information is available concerning the effectivenesses of higher doses in experimental models of spinal cord ischemia, thus, we cannot exclude the fact that a higher dose or a longer period of treatment may be required in spinal cord ischemia/reperfusion injury.
In a previous rat study, animals were given a single oral dose of thalidomide 2 h before the intravenous injection of LPS to assess its ability to suppress TNF-α expression in liver cells.24 However, after a single oral dose of 200 mg of thalidomide (as the US-approved capsule formulation) in healthy volunteers, absorption was found to be slow, and this resulted in a peak serum concentration (Cmax) of 1–2 mg/l at 3–4 h after administration, an absorption lag time of 30 min, an apparent elimination half-life of 6 h, and an apparent systemic clearance of 10 l/h.60 Therefore, in the present study, we injected thalidomide i.p. 3 h before ischemic insult, in view of its elimination half-life and the fact that absorption is faster by the i.p. route than by the oral route.
Prior to the present study, interim studies were performed to develop the experimental technique and to determine anesthetic maintenance doses; involving the use of ketamine hydrochloride and xylazine hydrochloride as anesthetic induction agents and the intravenous inject of additional xylazine hydrochloride for maintenance.
In conclusion, thalidomide administered i.p. before ischemic insult was found to reduce early phase ischemia/reperfusion injury of the spinal cord in rabbits. However, similar studies on various animals are required to obtain a reliable oversight of the effect of thalidomide on spinal cord ischemia/reperfusion injury in a clinical setting.
References
Svensson LG, Crawford ES, Hess KR, Coselli JS, Safi HJ . Experience with 1509 patients undergoing thoracoabdominal aortic operations. J Vasc Surg 1993; 17: 357–370.
Uno H, Matsuyama T, Akita H, Nishimura H, Sugita M . Induction of tumor necrosis factor-alpha in the mouse hippocampus following transient forebrain ischemia. J Cereb Blood Flow Metab 1997; 17: 491–499.
Liu T et al. Tumor necrosis factor-alpha expression in ischemic neurons. Stroke 1994; 25: 1481–1488.
Sairanen T et al. Evolution of cerebral tumor necrosis factor-alpha production during human ischemic stroke. Stroke 2001; 32: 1750–1758.
Cassada DC et al. Adensine A2A analogue ATL-146e reduces systemic tumor necrosing factor-alpha and spinal cord capillary platelet-endothelial cell adhesion molecule-1 expression after spinal cord ischemia. J Vasc Surg 2002; 35: 994–998.
Raje N, Anderson K . Thalidomide: a revival story. N Engl J Med 1999; 341: 1606–1609.
Moreira AL, Sampaio EP, Zmuidzinas A, Frindt P, Smith KA, Kaplan G . Thalidomide exerts its inhibitory action on tumor necrosis factor-alpha by enhancing mRNA degradation. J Exp Med 1993; 177: 1675–1680.
Murakami N et al. Hyperbaric oxygen therapy given 30 minutes after spinal cord ischemia attenuates selective motor neuron death in rabbits. Crit Care Med 2001; 29: 814–818.
Tarlov IM . Acute spinal cord compression paralysis. J Neurosurg 1972; 36: 10–20.
Pluta R, Lossinsky AS, Wisniewski HM, Mossakowski MJ . Early blood–brain barrier changes in the rat following transient complete cerebral ischemia induced by cardiac arrest. Brain Res 1994; 633: 41–52.
Kochanek PM, Hallenbeck JM . Polymorphonuclear leukocytes and monocytes/macrophages in the pathogenesis of cerebral ischemia and stroke. Stroke 1992; 23: 1367–1379.
Rumalla VK, Borah GL . Cytokines, growth factors, and plastic surgery. Plast Reconstr Surg 2001; 108: 719–733.
Lambertsen KL, Gregersen R, Finsen B . Microglial-macrophage synthesis of tumor necrosis factor after focal cerebral ischemia in mice is strain dependent. J Cereb Blood Flow Metab 2002; 22: 785–797.
Warren JS . Interleukins and tumor necrosis factor in inflammation. Crit Rev Clin Lab Sci 1990; 28: 37–59.
Ambrosio G, Tritto I . Reperfusion injury: experimental evidence and clinical implications. Am Heart J 1999; 138: S69–S75.
Gamble JR, Harlan JM, Klebanoff SJ, Vadas MA . Stimulation of the adherence of neutrophils to umbilical vein endothelium by human recombinant tumor necrosis factor. Proc Natl Acad Sci USA 1985; 82: 8667–8671.
Kang KJ . Mechanism of hepatic ischemia/reperfusion injury and protection against reperfusion injury. Transplant Proc 2002; 34: 2659–2661.
Donnahoo KK, Shames BD, Harken AH, Meldrum DR . Review article: the role of tumor necrosis factor in renal ischemia–reperfusion injury. J Urol 1999; 162: 196–203.
Strieter RM, Kunkel SL, Bone RC . Role of tumor necrosis factor-alpha in disease states and inflammation. Crit Care Med 1993; 21: S447–S463.
Shohami E, Ginis I, Hallenbeck JM . Dual role of tumor necrosis factor alpha in brain injury. Cytokine Growth Factor Rev 1999; 10: 119–130.
Hermann GE, Rogers RC, Bresnahan JC, Beattie MS . Tumor necrosis factor-alpha induces cFOS and strongly potentiates glutamate-mediated cell death in the rat spinal cord. Neurobiol Dis 2001; 8: 590–599.
Ishibashi N, Prokopenko O, Reuhl KR, Mirochnitchenko O . Inflammatory response and glutathione peroxidase in a model of stroke. J Immunol 2002; 168: 1926–1933.
Haslett PA, Corral LG, Albert M, Kaplan G . Thalidomide costimulates primary human T lymphocytes, preferentially inducing proliferation, cytokine production, and cytotoxic responses in the CD8+ subset. J Exp Med 1998; 187: 1885–1892.
Sampaio EP, Sarno EN, Galilly R, Cohn ZA, Kaplan G . Thalidomide selectively inhibits tumor necrosis factor alpha production by stimulated human monocytes. J Exp Med 1991; 173: 699–703.
Enomoto N, Takei Y, Hirose M, Kitamura T, Ikejima K, Sato N . Protective effect of thalidomide on endotoxin-induced liver injury. Alcohol Clin Exp Res 2003; 27: 2S–6S.
Turk BE, Jiang H, Liu JO . Binding of thalidomide to alpha1-acid glycoprotein may be involved in its inhibition of tumor necrosis factor alpha production. Proc Natl Acad Sci USA 1996; 93: 7552–7556.
Kim YS, Kim JS, Jung HC, Song IS . The effects of thalidomide on the stimulation of NF-kappaB activity and TNF-alpha production by lipopolysaccharide in a human colonic epithelial cell line. Mol Cells 2004; 17: 210–216.
May MJ, Ghosh S . Signal transduction through NF-kappaB. Immunol Today 1998; 19: 80–88.
Lokensgard JR, Hu S, Fenema EM, Sheng WS, Peterson PK . Effect of thalidomide on chemokine production by human microglia. J Infect Dis 2000; 182: 983–987.
Keifer JA, Guttridge DC, Ashburner BP, Baldwin Jr AS . Inhibition of NF-kappa B activity by thalidomide through suppression of IkappaB kinase activity. J Biol Chem 2001; 276: 22382–22387.
Rowland TL, McHugh SM, Deighton J, Ewan PW, Dearman RJ, Kimber I . Differential effect of thalidomide and dexamethasone on the transcription factor NF-kappaB. Int Immunopharmacol 2001; 1: 49–61.
Majumdar S, Lamothe B, Aggarwal BB . Thalidomide suppresses NF-kappaB activation induced by TNF and H2O2, but not that activated by ceramide, lipopolysaccharides, or phorbol ester. J Immunol 2002; 168: 2644–2651.
Bauditz J, Wedel S, Lochs H . Thalidomide reduces tumor necrosis factor alpha and interleukin 12 production in patients with chronic active Crohn's disease. Gut 2002; 50: 196–200.
Hori K, Mihich E, Ehrke MJ . Role of tumor necrosis factor and interleukin-1 in gamma-interferon-promoted activation of mouse tumoricidal macrophages. Cancer Res 1989; 49: 2606–2614.
Hattori A et al. Tumor necrosis factor stimulates the synthesis and secretion of biologically active nerve growth factor in non-neuronal cells. J Biol Chem 1993; 268: 2577–2582.
Matsuyama T, Shimizu S, Nakamura H, Michishita H, Tagaya M, Sugita M . Effects of recombinant superoxide dismutase on manganese superoxide dismutase gene expression in gerbil hippocampus after ischemia. Stroke 1994; 25: 1417–1424.
Boone E et al. Structure/Function analysis of p55 tumor necrosis factor receptor and fas-associated death domain. Effect on necrosis in L929sA cells. J Biol Chem 2000; 275: 37596–37603.
Smith CA, Farrah T, Goodwin RG . The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell 1994; 76: 959–962.
Glazner GW, Mattson MP . Differential effects of BDNF, ADNF9, and TNFalpha on levels of NMDA receptor subunits, calcium homeostasis, and neuronal vulnerability to excitotoxicity. Exp Neurol 2000; 161: 442–452.
Bruce AJ et al. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat Med 1996; 2: 788–794.
Nawashiro H, Tasaki K, Ruetzler CA, Hallenbeck JM . TNF-alpha pretreatment induces protective effects against focal cerebral ischemia in mice. J Cereb Blood Flow Metab 1997; 17: 483–490.
Scherbel U et al. Differential acute and chronic responses of tumor necrosis factor-deficient mice to experimental brain injury. Proc Natl Acad Sci USA 1999; 96: 8721–8726.
Baeuerle PA, Henkel T . Function and activation of NF-kappaB in the immune system. Annu Rev Immunol 1994; 12: 141–179.
Marchetti L, Klein M, Schlett K, Pfizenmaier K, Eisel UL . Tumor necrosis factor (TNF)-mediated neuroprotection against glutamate-induced excitotoxicity is enhanced by N-methyl-D-aspartate receptor activation. Essential role of a TNF receptor 2-mediated phosphatidylinositol 3-kinase-dependent NF-kappaB pathway. J Biol Chem 2004; 279: 32869–32881.
Sawada M, Kondo N, Suzumura A, Marunouchi T . Production of tumor necrosis factor-alpha by microglia and astrocytes in culture. Brain Res 1989; 491: 394–397.
Sharif SF, Hariri RJ, Chang VA, Barie PS, Wang RS, Ghajar JB . Human astrocyte production of tumour necrosis factor-alpha, interleukin-1 beta and interleukin-6 following exposure to lipopolysaccharide endotoxin. Neurol Res 1993; 15: 109–112.
Shohami E, Novikov M, Bass R, Yamin A, Gallily R . Closed head injury triggers early production of TNF-alpha and IL-6 by brain tissue. J Cereb Blood Flow Metab 1994; 14: 615–619.
Matsumoto S et al. The temporal profile of the reaction of microglia, astrocytes, and macrophages in the delayed onset paraplegia after transient spinal cord ischemia in rabbits. Anesth Analg 2003; 96: 1777–1784.
Rupalla K, Allegrini PR, Sauer D, Wiessner C . Time course of microglia activation and apoptosis in various brain regions after permanent focal cerebral ischemia in mice. Acta Neuropathol 1998; 96: 172–178.
Peterson PK et al. Thalidomide inhibits tumor necrosis factor-alpha production by lipopolysaccharide- and lipoarabinomannan-stimulated human microglial cells. J Infect Dis 1995; 172: 1137–1140.
Sakurai M, Aoki M, Abe K, Sadahiro M, Tabayashi K . Selective motor neuron death and heat shock protein induction after spinal cord ischemia in rabbits. J Thorac Cadiovasc Surg 1997; 113: 159–164.
Kojima M et al. Effects of antioxidative agents on apoptosis induced by ischemia-reperfusion in rat intestinal mucosa. Ailment Pharmacol Ther 2003; 18: 139S–145S.
Lian X-Y, Stringer JL . Energy failure in astrocytes increases the vulnerability of neurons to spreading depression. Eur J Neurosci 2004; 19: 2446–2454.
Greiner C et al. Acute protective effect of nimodipine and dimethyl sulfoxide against hypoxic and ischemic damage in brain slices. Brain Res 2000; 887: 316–322.
Kiyoshima T et al. Lack of evidence for apoptosis as a cause of delayed onset paraplegia after spinal cord ischemia in rabbits. Anesth Analg 2003; 96: 839–846.
Muriel P et al. Thalidomide ameliorates carbon tetrachloride induced cirrhosis in the rat. Eur J Gastroenterol Hepatol 2003; 15: 951–957.
Lentzsch S et al. Immunomodulatory analogs of thalidomide inhibit growth of Hs Sultan cells and angiogenesis in vivo. Leukemia 2003; 17: 41–44.
Yamamoto S, Cooper DK . An investigation of the effect of thalidomide on anti-gal antibody production in baboons. Xenotransplantation 2003; 10: 470–474.
Deng L, Ding W, Granstein RD . Thalidomide inhibits tumor necrosis factor-alpha production and antigen presentation by Langerhans cells. J Invest Dermatol 2003; 121: 1060–1065.
Teo SK et al. Clinical pharmacokinetics of thalidomide. Clin Pharmacokinet 2004; 43: 311–327.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lee, CJ., Kim, KW., Lee, HM. et al. The effect of thalidomide on spinal cord ischemia/reperfusion injury in a rabbit model. Spinal Cord 45, 149–157 (2007). https://doi.org/10.1038/sj.sc.3101931
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101931
Keywords
This article is cited by
-
Transiently lowering tumor necrosis factor-α synthesis ameliorates neuronal cell loss and cognitive impairments induced by minimal traumatic brain injury in mice
Journal of Neuroinflammation (2015)
-
Intraperitoneal Injection of Thalidomide Attenuates Bone Cancer Pain and Decreases Spinal Tumor Necrosis Factor-α Expression in a Mouse Model
Molecular Pain (2010)