Abstract
Study design
Animal study.
Objectives
Explore the neuroprotective effect of remote limb ischemic postconditioning (Post C) in spinal cord ischemic reperfusion injury (SCII) and related mechanisms.
Setting
Anesthesiology Laboratory of Southwest Medical University.
Methods
We established a rabbit SCII model and processed it with Post C. To evaluate the neural function, spinal cord tissue was taken 48 h later, normal neurons were evaluated by HE staining, and the expression of ATP-sensitive potassium channel (KATP) marker molecule Kir6.2 was detected by Western blot. Immunofluorescence detection of spinal cord Iba-1 expression, ELISA detection of M1 type microglia marker iNOS and M2 type microglia marker Arg, and Western blot detection of NF-κB and IL-1β expression. Through these experiments, we will explore the protective effect of Post C in SCII, observe the changes in the protective effect after using KATP blockers, and verify that Post C can play a neuroprotective effect in SCII by activating KATP.
Results
We observed that Post C significantly improved exercise ability and the number of spinal motor neurons in the SCII model. Microglia are activated and expression of M1 microglia in the spinal cord was decreased, while M2 was increased. This neuroprotective effect was reversed by the nonspecific KATP inhibitor.
Conclusion
Post C has a neuroprotective effect on SCII, and maybe a protective effect produced by activating KATP to regulate spinal microglia polarization and improve neuroinflammation.
Similar content being viewed by others
Introduction
Severe ischemia can cause different degrees of damage to the spinal cord, and subsequent spinal cord ischemic reperfusion injury (SCII) is a further fatal process [1,2,3]. Applying a transient sublethal ischemic load before fatal ischemia induces tolerance to subsequent ischemic injury and is known as ischemic preconditioning [4, 5], which shows a strong neuroprotective effect [6]. Studies have shown that the opening of KATP channels is an important mechanism underlying the neuroprotective effects of ischemic preconditioning and reduces the degree of spinal cord injury during limb ischemia-reperfusion [7, 8]. However, the clinic occurrence of the ischemic disease is highly variable, and the occurrence of ischemia cannot be accurately predicted at present. Therefore, the concept of ischemic postconditioning (Post C) has been proposed [9] in which two min before opening of the abdominal aorta, a double lower limb popliteal hemostatic belt 200 mmHg was pressed for 5 min, loosened for 5 min, and repeated three times, which has a protective effect on ischemia-reperfusion of important organs, such as the heart and brain [10, 11]. However, whether Post C reduces SCII damage has not yet been reported.
ATP-sensitive potassium (KATP) channels are widely expressed in the inner and outer mitochondrial membranes of the nervous and cardiovascular systems [12] and play an important role in hypoxia [13]. In cerebral ischemic disease, upregulating expression of KATP channels reduces infarct size and improves nerve function, and KATP channel inhibitors reverse this protective effect [14]. The study has found that KATP channels are involved in the protective process of ischemic preconditioning during spinal cord ischemia-reperfusion injury. The possible mechanism is that after activation of KATP channels, intracellular K+ outflow causes hyperpolarization of nerve cell membranes, and Ca2+ intracellular transport is reduced, causing intracellular Ca2+ concentration to decrease [15] and reducing cell damage caused by ischemia and excessive hypoxia-induced glutamate release [16]. In spinal cord ischemia and perfusion injury, ion overload causes intracellular edema, which is an important cause of cell membrane injury. How to reduce ion overload is an important research target at present, and KATP channels play an important role in regulating cell ion transport [17]. In addition, KATP is widely expressed on the cell membrane of microglia cells and plays an important role in the activation of microglia, thereby regulating microglia-related neuroinflammation [18]. Polarization of microglial cells in ischemia-reperfusion injury is gaining increasing attention [19]. Overactivated microglial (M1) cells undergo an inflammatory cascade and release large amounts of NF-κB, IL-1β. Inflammatory substances, with the occurrence of inflammatory damage, induce the polarization of microglia to the M2 state. M2 microglia can clear inflammatory substances such as IL-4 to produce a repairing effect. Balancing the polarization of microglia is an important target for mitigating SCII damage [20]. The existence of KATP channels in microglia has been confirmed, and these KATP channels have a regulatory effect on the activation of microglia [21]. After ischemia and hypoxia, expression of KATP channels in microglia is increased, and expression of SUR1 and SUR2 is detected in activated microglia but not in resting microglia. The KATP channel opener can reduce rotenone-induced microglial activation and downregulates the expression TNF-α and cyclooxygenase-2 (COX-2) mRNA [22]. Based on the existing evidence, we hypothesized that Post C has a neuroprotective effect in SCII that is achieved by activating KATP channels to regulate the polarization of microglia.
In this study, we established a SCII model and observed the neuroprotective effect of Post C treatment on the SCII model, and discussed whether Post C regulates microglia-related neuroinflammation through KATP channels and produces neuroprotective effect
Materials and methods
Animals and groups
Sixty-four healthy adult male New Zealand white rabbits (1.2–1.5 kg) were provided by the Animal Experimental Center of Southwest Medical University, Keeping the day and night alternating and the temperature is 22 °C. All the rabbits were randomly divided into four groups by means of random sequence (Sham, SCII, Post C, and GLI). Sham group was control group, other three groups of rabbits had the abdomen aorta clamped for 25 min and then were re-perfused. Ischemic postconditioning was performed in Post C, and GLI groups. The GLI group was given intraperitoneal injection of glibenclamide (1 mg/kg) 10 min before spinal cord ischemia (Fig. 1A).
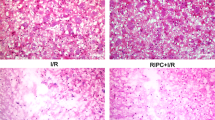
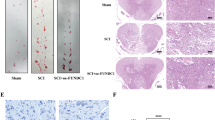
A Processing timeline of experimental plotted. B Effects of glibenclamide (0.1–2 mg/kg, i.p.) on blood glucose levels (mmol/L), data represent Mean ± S.E.M (ANOVA, *P < 0.05 indicates comparison with saline; #P < 0.05 indicates comparison with vehicle). C H&E staining of spinal cord. The yellow arrow indicates the motor neurons in the dorsal horn of the spinal cord. The number of motor neurons in the SCII group was significantly reduced. Post C reduced the loss of motor neurons, and KATP blocker weakened the neuronal protective effect of Post C. D–G Nerve function score. data represent Mean ± S.E.M (Kruskal–Wallis, *P < 0.05 indicates comparison with Sham; #P < 0.05 comparison with SCII, &P < 0.05 comparison with Post C). H Western blot results of KATP-related protein Kir6.2, based on quantification using Image J software, date are shown as Mean ± S.E.M (ANOVA, *P < 0.05 indicates comparison with Sham; #P < 0.05 comparison with SCII, &P < 0.05 comparison with Post C).
Determination of KATP inhibitor dose (each group n = 5)
The nonselective KATP channel inhibitor glibenclamide was diluted in vehicle (20% DMSO-50% alcohol (96%)-30% saline). Blood glucose levels were measured to determine the lowest effective dose of glyburide involved in pancreatic KATP channel inhibition. The glibenclamide dose gradient was set to 0.1, 1, and 2 mg/kg. We found that the vehicle significantly increased blood glucose of rabbits compared to the blank control (P < 0.05), the 1 mg/kg dose of glibenclamide lowered blood glucose levels by 50% compared to vehicle (P < 0.05), and 10 mg/kg dose did not further improve this efficacy. We finally determined that the minimum effective dose of glibenclamide in the vehicle was 1 mg/kg (Fig. 1B).
Establishment of the SCII model
A classic ZIVIN method [23] was used to establish a rabbit spinal cord ischemia-reperfusion model. Pentobarbital sodium provides adequate anesthesia and relieves pain in animals. The abdominal aorta was clamped with a nondamaged arterial clip for 25 min and then reopened. The waveform of the femoral artery disappeared during occlusion. The pressure monitoring value DAP ≤ 10 mmHg to both lower limbs cyanosis indicates that the abdominal aorta is completely blocked. After 25 min, the arterial clamp was released to open the abdominal aorta, and femoral artery pressure waveform was restored. We recorded the vital signs of the rabbits before spinal cord ischemia, 15 min after ischemia, and 15 min after reperfusion, indicating that the Post C treatment is safe (Supplementary Data 1).
Post C surgical process
The two ends of the two baby-type blood pressure cuffs were connected to the pressure gauge and the air pump. After confirming that there was no air leakage, the two blood pressure cuffs were looped to 1/3 of the rabbit popliteal fossa, and the air pump knob was tightened. Before resuming abdominal aortic infusion, the inflator was pressurized on both sides at the same time until the pressure gauge showed a pressure of 200 mmHg. After maintaining the pressure for 5 min, the pump knob was unscrewed and the cuff was loosened and deflated to 0 mmHg for 5 min, which was repeated three times.
Neurological score (each group n = 8)
The modified Tarlov scoring method was used to evaluate motor function of the hind limbs of the rabbits at 4, 12, 24, and 48 h after spinal cord ischemia and reperfusion. Modified Tarlov scoring criteria were computed as follows: 0 points: no perceptible lower limb movement; one point: perceptible hind limb joint voluntary movement; 2 points: hind limbs can move freely but the animal cannot stand; three points: animal can stand but cannot walk; four points: hind limb function is completely restored, and animal can walk normally. 0–1 point is rated as paraplegic. Behavioral studies are conducted at 10:00–12:00 a.m. The ethology tests were conducted by researchers who did not know what the groups were, and were presented with “Group 1, 2, 3, 4”.
Motor neuron count in anterior spinal cord (each group n = 8)
After 48 h of ischemia-reperfusion, animals were anesthetized, and the lumbar spinal cord tissues (L5-7) were collected. Specimens were obtained and fixed for 24 h with 10% neutral formaldehyde. Spinal cord tissue was embedded, sliced thick (6 μm) and HE stained to make 3 HE stained sections from each animal and observed under 200x light microscopy to observe histopathological changes of the spinal cord.
Immunofluorescence (each group n = 5)
Fix for 30 min with 4% tissue cell fixative. Soak in Triton for 15 min, wash and block with 1% BSA at room temperature for 1 h, add Iba-1 primary antibody, add fluorescent secondary antibody and incubate for 1 h at 4 °C overnight, discard the secondary antibody, add DAPI, and add anti-fluorescence quencher. Cover film. Observe under a fluorescence microscope.
ELISA: detection of inducible NOS (iNOS) and arginase (Arg) expression in spinal cord tissue (each group n = 5)
As soluble mediators produced by different types of macrophages, iNOS, and Arg can indirectly reflect two different polarization states of microglia through their expression levels. Taking an appropriate amount of tissue blocks in prechilled PBS, tissue was homogenized, and the supernatant was collected. The rabbit iNOS Elisa kit (Shanghai Qiaodu Biotech, China) and the rabbit Arg Elisa kit (Shanghai Qiaodu Biotech, China) were used to detect polarization status of spinal cord microglia. Standard flat preparations were created according to the instructions (iNOS:0,2,4,8,16,32 U/L; Arg: 0,1,2,4,8,16 U/L), and the OD of each well was measured at 450 nm within 15 min.
West blotting: detection of Kir6.2, NF-κB and IL-1β expression in spinal cord tissue (each group n = 5)
RIPA lysis buffer (Beyotime, Shanghai, China) was added to each sample, which included the following ingredients: protease inhibitor (Beyotime, Shanghai, China), phosphatase inhibitor (Beyotime, Shanghai, China) was added at 100:1:1 to lyse spine tissues for 60 min. Fully homogenized tissue and supernatants were then collected. The BCA method (Beyotime, Shanghai, China) was used to determine protein concentrations for each sample. Subsequently, protein samples were separated by 10% SDS polyacrylamide gel and transferred onto PVDF membranes (IPVH00010, Millipore, Billerica, MA, USA). Membranes were blocked with 10% nonfat milk for 60 min at room temperature, followed by the addition of primary antibodies (Rabbit Anti-Kir6.2/BIR antibody (1:1000, Abcam, US); Anti- NF-κB p65 (phosphor-S536) (1:1000 Abcam, US); Anti-IL-1 beta (1:1000, Abcam, US); Rabbit Anti-GAPDH antibody (1:1000 Abcam, US)) at 4 °C overnight. Secondary antibody (IgG H&L (HRP), 1:5000, Proteintech, China) was incubated with the membranes at 37 °C for 60 min. Electrochemical luminescence (Tambo, Chengdu, China) was used to display bands.
Statistical analysis
SPSS 24.0 statistical software was used for analysis. Nonparametric rank-sum test (Kruskal–Wallis test) was used to compare neurological function scores. One-factor analysis of variance (ANOVA) was used to compare the expressions of Iba-1, Kir6.2, iNOS, Arg-1, p-NF-κB, and IL-β. P < 0.05 indicates statistical significance.
Results
Post C exerts strong neuroprotective effects in SCII, and these protective effects are reversed by the KATP channel inhibitor glibenclamide
First, by observing spinal cord motor neurons, we have observed the neuroprotective effect of Post C (Fig. 1C). Second, the modified Tarlov scoring method was used to evaluate motor function of the hind limbs of rabbits 4, 12, 24, and 48 h after spinal cord ischemia and reperfusion. Results showed that nerve function scores of the Post C can improve the neurological score of SCII rabbits, and this effect can be partially reversed by glibenclamide at all four time points (Fig. 1D–G). From these results, we once again verified that Post C has a neuroprotective effect on SCII, and KATP channel blockers reverse this neuroprotective effect, indicating that the neuroprotective effect of Post C maybe achieved through activation of KATP channels. We detected Kir6.2, the marker molecule of KATP channels by Western blot. We found that the expression of SCII, the Kir6.2 was reduced, while Post C treatment could increase its expression, and it could be reversed by glibenclamide (Fig. 1H).
Post C activates KATP channels that play a neuroprotective role by regulating microglia polarization
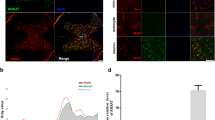
Next, we explored the mechanism of KATP channel activation and its neuroprotective role in SCII. KATP channels in the spinal cord are highly expressed in microglia. We speculate that Post C may activate the neuroprotective effects of KATP channels on microglia. Immunofluorescence showed that Post C can significantly reduce the expression of spinal cord Iba-1, while the addition of glibenclamide partially reversed this effect (Fig. 2A, B). We detected the expression of the M1 state microglia marker molecule iNOS and the M2 state microglia marker molecule Arg in three groups of rabbit spinal cords by enzyme-linked immunoassay (ELISA). We found that 48 h after ischemia-reperfusion, iNOS expression in the Post C-treated rabbit spinal cord was decreased compared to that in control rabbits, while Arg expression was increased, and administration of glibenclamide reversed this microglial polarization effect (Fig. 2C, D). To further prove that Post C may activate neuroprotection through KATP channels that regulate the polarization status of microglia, we examined the inflammatory factors NF-κB and IL-1β, which are closely related to microglia. Surprisingly, we found that Post C significantly reduces the expression of NF-κB and IL-1β, and expression of these inflammatory factors was reversed by KATP channel blockers (Fig. 3A–B). From these results, we conclude that the neuroprotective effects of Post C-activated KATP channels in SCII are related to microglia and maybe achieved by regulating microglial polarization status. However, the specific mechanism may require further research.
A Iba-1 immunofluorescence staining 48 h after ischemia-reperfusion (magnification 200×, green is Iba-1, blue is DAPI). B Iba-1 fluorescence quantification, analyzed by Image software, data represent Mean ± S.E.M (ANOVA, *P < 0.05 indicates comparison with Sham; #P < 0.05 comparison with SCII, &P < 0.05 comparison with Post C). C, D M1 type microglia marker iNOS and M2 type microglia marker Arg-1 are expressed. data represent Mean ± S.E.M (ANOVA, *P < 0.05 indicates comparison with Sham; #P < 0.05 comparison with SCII, &P < 0.05 comparison with Post C).
A Western blot results of p-NF-κB, based on quantification using Image J software, showed as Mean ± S.E.M (ANOVA, ANOVA, *P < 0.05 indicates comparison with Sham; #P < 0.05 comparison with SCII, &P < 0.05 comparison with Post C). B Western blot results of IL-1β, showed as Mean ± S.E.M (ANOVA, ANOVA, *P < 0.05 indicates comparison with Sham; #P < 0.05 comparison with SCII, &P < 0.05 comparison with Post C). C Schematic diagram of the protective effect of Post C on SCII through regulating the polarization state of microglia.
Discussion
Spinal nerve cells are extremely sensitive to injury. Once nerve cells are injured, spinal cord function is difficult to restore, and disability is extremely high. SCII is an important cause of spinal cord injury [1, 24]. The importance of exploring effective neuroprotective methods is highlighted, but unfortunately, the mechanism of spinal cord injury caused by SCII has not yet been fully elucidated. This study aimed to identify new therapeutic targets for SCII and further explore the specific mechanism of SCII. This study verified the neuroprotective effect of Post C in SCII by establishing an SCII model, demonstrating the neuroprotective role of Post C in SCII occurs through activation of KATP channels that regulate microglial polarization.
Post C refers to the measures of carrying out multiple short and repeated ischemia and reperfusion of the distal limbs after ischemic injury to important organs, initiation endogenous protection of the body, and protecting ischemic organs [9]. A previous study in male mice performed coronary artery occlusion for 45 min, at the beginning of the perfusion period, three cycles of ischemia and 5 min of reperfusion were performed on the left hind limb. After 2 h of reperfusion, the area of myocardial infarction, myocardial enzyme release, and cells in Post C mouse apoptosis were reduced [25]. Some studies have reported that giving three repeated ischemia-reperfusion treatments of 5 min to clip the femoral artery significantly reduces the volume of cerebral infarction in the limb posttreatment group, indicating that transient and repeated limb posttreatment reduces cerebral ischemia-reperfusion injury [11]. In a randomized clinical trial in ischemic stroke patients, the experimental group was given four cycles of 5 min cuff inflation ischemia-reperfusion limb ischemia treatment, and the posttreatment group had a smaller infarct size than the control group. Post C reduces nerve cell damage in stroke patients. Subsequent studies found that Post C also improves prognosis and cognitive impairment after cerebral infarction [26]. Post C exhibited a protective effect on ischemia-reperfusion injury to the heart and brain. Through our experiments, we verified that Post C also demonstrated a neuroprotective effect, and to our knowledge, this result is innovative. Judging from our experiments, there was no obvious impact on the vital signs of animals during Post C processing, suggesting that this processing is a relatively safe method.
KATP channels are a kind of voltage-independent special potassium channel that uses intracellular ATP/ADP levels as the gating factor, coupling cell electrical activity and metabolism and playing an important role in various physiological and pathological processes. KATP channels are composed of inwardly rectifying potassium channel subunits and sulfonylurea receptors (SURs). Therefore, KATP channels can be activated by a variety of potassium channel openers and inhibited by sulfonylurea complexes [13]. Studies have reported that KATP channel openers significantly reduce infarct size after cerebral ischemic injury and reduce the damage to nerve function, and KATP channel antagonists offset this protective effect [14]. One study found that KATP channels are involved in the protective process of ischemic preconditioning against spinal cord ischemia-reperfusion injury. The possible mechanism is that after activation of KATP channels, hyperpolarization of the nerve cell membrane caused by K+ outflow in the cell causes Ca2+ to enter the cell. Then, transport decreases and intracellular Ca2+ concentration decreases, thereby reducing cell damage caused by ischemia and hypoxia-induced glutamate over release [15]. Based on studying the protective effect of Post C on spinal cord ischemic injury, this experiment explored whether KATP channels are also involved in this process.
The KATP channel is closely related to the activation of microglia. After ischemia and hypoxia, the expression of KATP channels in microglia will increase. In the early stages of ischemia-reperfusion injury, the KATP blocker glibenclamide promotes microglial activation, which enhances the phagocytic capacity of microglia and the release of NF-κB, which promotes the removal of cell debris and cytotoxic substances and inhibits neutrophils from further releasing cytotoxic substances, thereby reducing nerve damage [19]. In the later period, microglial cells continue to be activated, releasing a large number of inflammatory cytotoxic factors, and stimulating the inflammatory response will increase damage to neurons [20]. Our results demonstrated that Post C treatment inhibited the overactivation of microglia to produce a neuroprotective effect, and KATP blockers block this protective effect, which is contrary to the results of Morrison et al. The primary point is that the time point we detected was 48 h after ischemia-reperfusion. Inhibition of KATP channels during the early stage of ischemia-reperfusion may appear to be short-lived and inhibit the activation of microglia, but as time moves on, activation of KATP channels can accelerate M2 type transition. Microglial cells are activated, thereby fighting the nerve damage of M1 type glial cells. The damage of SCII involves a long-term inflammatory cascade reaction [21]. Imbalanced microglia activation aggravates nerve damage. Reasonable regulation of the polarization state is an important target for neuroprotection. Our results show that Post C activates KATP channels to regulate the state of microglia polarization (Fig. 3C). Unfortunately, we did not dynamically monitor the polarization status of microglia at different time points, which maybe a problem that needs further investigation in the future.
Clinically, the occurrence of ischemic injury is difficult to predict. Timely intervention is an important strategy to reduce further reperfusion injury. Post C is a kind of ischemic injury after processing method, it can occur in spinal cord ischemia-reperfusion after the purpose of the intervention [3], but our results can only be concluded based on animal experiments, further may require large sample randomized controlled clinical trials explore Post C for spinal cord ischemia-reperfusion injury of protection. On the other hand, the use of KATP agonists is another research direction to enhance the neuroprotective effect of Post C.
Conclusion
In conclusion, our study verified that Post C exerts a neuroprotective effect in SCII. This neuroprotective effect maybe achieved by activating KATP channels to regulate microglial polarization status. However, the specific control mechanisms governing this process needs further study.
Data availability
Raw data related to the paper can be obtained by email from the corresponding author.
References
Li M, Zhao K, Ruan Q, Meng C, Yin F. The transcription factor Foxd3 induces spinal cord ischemia-reperfusion injury by potentiating microRNA-214-dependent inhibition of Kcnk2. Exp Mol Med. 2020;52:118–29.
Jin N, Fang B, Li Z, Tian A. Exogenous activation of cannabinoid-2 receptor modulates TLR4/MMP9 expression in a spinal cord ischemia reperfusion rat model. J Neuroinflammation. 2020;17:101.
Farjah GH, Mohammad PM, Khadem-Ansari MH, Karimipour M, Pourheidar BL. Spinacia oleracea, Protective effect of aqueous spinach extract on spinal cord ischemia-reperfusion injury in rats. Vet Res Forum. 2018;9:187–91.
Jung KW, Kang J, Kwon HM, Moon YJ, Jun IG, Song JG, et al. Effect of remote ischemic preconditioning conducted in living liver donors on postoperative liver function in donors and recipients following liver transplantation: a randomized clinical trial. Ann Surg. 2020;4:646–53.
Tang YL, Zhu W, Cheng M, Chen L, Zhang J, et al. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ Res. 2009;104:1209–16.
Sawashita Hirata YN, Yoshikawa Y, Terada H, Tokinaga Y, Yamakage M. Remote ischemic preconditioning reduces myocardial ischemia-reperfusion injury through unacylated ghrelin-induced activation of the JAK/STAT pathway. Basic Res Cardiol. 2020;115:50.
Sun HS, Xu B, Chen W, Xiao A, Turlova E, Alibraham A, et al. Neuronal K(ATP) channels mediate hypoxic preconditioning and reduce subsequent neonatal hypoxic-ischemic brain injury. Exp Neurol. 2015;263:161–71.
Hu X, Yang Z, Yang M, Qian J, Cahoon J, Xu J, et al. Remote ischemic preconditioning mitigates myocardial and neurological dysfunction via K(ATP) channel activation in a rat model of hemorrhagic shock. Shock 2014;42:228–33.
Okorie MI, Bhavsar DD, Ridout D. Postconditioning protects against human endothelial ischaemia-reperfusion injury via subtype-specific KATP channel activation and is mimicked by inhibition of the mitochondrial permeability transition pore. Eur Heart J. 2011;32:1266–74.
Loukogeorgakis SP, Williams R, Panagiotidou AT, Kolvekar SK, Donald A. Transient limb ischemia induces remote preconditioning and remote postconditioning in humans by a KATP-channel dependent mechanism. Circulation 2007;116:1386–95.
Wang Z, Wu L, Xu J, Gao J. Limb ischemic postconditioning alleviates postcardiac arrest syndrome through the inhibition of mitochondrial permeability transition pore opening in a porcine model. Biomed Res Int. 2020;2020:9136097.
Foster MN, Coetzee WA. KATP channels in the cardiovascular system. Physiol Rev. 2016;96:177–252.
Li N, Wu JX, Ding D, Cheng J, Gao N, Chen L. Structure of a pancreatic ATP-sensitive potassium channel. Cell 2017;168:101–110.e110.
Al-Karagholi MA, Hansen JM, Guo S, Olesen J. Opening of ATP-sensitive potassium channels causes migraine attacks: a new target for the treatment of migraine. Brain 2019;142:2644–54.
Levin SG, Godukhin OV. Comparative roles of ATP-sensitive K+ channels and Ca2+-activated BK+ channels in posthypoxic hyperexcitability and rapid hypoxic preconditioning in hippocampal CA1 pyramidal neurons in vitro. Neurosci Lett. 2009;461:90–94.
Kim KO, Choe G, Chung SH, Kim CS. Delayed pharmacological pre-conditioning effect of mitochondrial ATP-sensitive potassium channel opener on neurologic injury in a rabbit model of spinal cord ischemia. Acta Anaesthesiol Scand. 2008;52:236–42.
Alber TL, Gonzale S. Ca2+ release from the sarcoplasmic reticulum is required for sustained TRPM4 activity in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol. 2010;299:279–88.
Du RH, Sun HB, Hu ZL, Lu M, Ding JH, Hu G. Kir6.1/K-ATP channel modulates microglia phenotypes: implication in Parkinson’s disease. Cell Death Dis. 2018;9:404–17.
Yang YW, Wang YL, Lu JK, Tian L. Delayed xenon post-conditioning mitigates spinal cord ischemia/reperfusion injury in rabbits by regulating microglial activation and inflammatory factors. Neural Regen Res. 2018;13:510–7.
Niida-Kawaguchi M, Kakita A, Noguchi N, Kazama M, Masui K. Soluble iron accumulation induces microglial glutamate release in the spinal cord of sporadic amyotrophic lateral sclerosis. Neuropathology 2020;40:152–66.
Zhou F, Yao HH, Wu JY, Ding JH, Sun T, Hu G. Opening of microglial K(ATP) channels inhibits rotenone-induced neuroinflammation. J Cell Mol Med. 2008;12:1559–70.
Zhou F, Wu JY, Sun XL, Yao HH. Iptakalim alleviates rotenone-induced de ation of dopaminergic neurons through inhibiting microglia-mediated neuroinflammation. Neuropsychopharmacology 2007;32:2570–80.
Zivin JA, DeGirolami U. Spinal cord infarction: a highly reproducible stroke model. Stroke 1980;11:200–2.
Tokuda Y, Fujimoto K, Narita Y, Mutsuga M. Spinal cord injury following aortic arch replacement. Surg Today. 2020;50:106–13.
Ovize M, Mewton N. Interventional cardiology: ischaemic POSTconditioning-a long harvest for a little corn. Nat Rev Cardiol. 2014;11:8–10.
Engstrøm N, Kelbæk H, Helqvist S, Høfsten DE, Kløvgaard L. Effect of ischemic postconditioning during primary percutaneous coronary intervention for patients with ST-segment elevation myocardial infarction: a randomized clinical trial. JAMA Cardiol. 2017;2:490–7.
Acknowledgements
Thanks to Dr. Jianguo Feng from the Anesthesiology Laboratory of Southwest Medical University for supporting this study. This study was supported by Zigong Science and Technology Bureau (2021YLSF16).
Author information
Authors and Affiliations
Contributions
JF designed the experiment and carried out the experiment; GM was involved in experiment design, experiment implementation, paper writing, data management; XL and CO participated in the implementation of part of the experiment, while JZ was responsible for the whole experiment and the final authorization.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by the Animal Ethics Committee of Southwest Medical University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Fu, J., Mu, G., Liu, X. et al. Ischemic postconditioning reduces spinal cord ischemia-reperfusion injury through ATP-sensitive potassium channel. Spinal Cord 60, 326–331 (2022). https://doi.org/10.1038/s41393-021-00714-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-021-00714-5