Abstract
Study design:
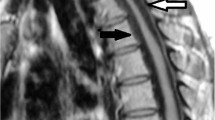
Immnunohistochemical staining of the thickened posterior longitudinal ligament of the cervical spine.
Objectives:
To clarify the histological characteristics of hypertrophy of the posterior longitudinal ligament (HPLL) of the cervical spine and the relationship between HPLL and ossification of the posterior longitudinal ligament (OPLL).
Setting:
Aichi Medical University, Aichi, Japan.
Methods:
Eight specimens of HPLL and two of OPLL were obtained during anterior decompressive surgery on the cervical spine from patients with myelopathy. Hematoxylin and eosin staining, alcian blue staining and immunohistochemical staining with antibodies against bone morphogenetic protein (BMP), transforming growth factor (TGF)-β, proliferating cell nuclear antigen (PCNA), alkaline phosphatase (ALP) and osteopontin (OPN) were carried out on the specimens.
Results:
HPLL showed hyalinoid degeneration, the proliferation of chondrocytes and fibroblast-like spindle cells, infiltration of vessels and small ossification. In four cases, chondroid tissue was prominent with chondrocytes, which were expressed by ALP and OPN. The cells in HPLL were weakly or moderately stained by BMP, TGF-β and PCNA. Their expression was similar to that of OPLL. Immunohistochemical staining was negative for all cells in the control cases.
Conclusions:
Histological and biochemical evidence supports the hypothesis that HPLL transforms into OPLL. The positive expression of BMP and TGF-β in HPLL cells of myelopathic patients, and their similarity to OPLL, suggest that these cells have the potential to differentiate into osteogenic cells. Of note, neither BMP nor TGF-β was demonstrated in the PLL of control subjects. Furthermore, the expression of chondrocytes by ALP and OPN in cartilage-prominent HPLL suggests that the cartilage can be replaced by new bone.
Similar content being viewed by others
Introduction
Some authors1, 2 proposed that hypertrophy of the posterior longitudinal ligament (HPLL) might be a prestage of ossification of the posterior longitudinal ligament (OPLL). HPLL is defined as abnormal thickening of the posterior longitudinal ligament (PLL) without prominent ossification.1 However, the progression of ectopic ossification in HPLL tissue and its transformation into OPLL has not been histologically or immunohistochemically confirmed. Bone morphogenetic protein (BMP), an osteogenic protein, induces new bone formation in ectopic sites by endochondral ossification.3, 4 Transforming growth factor (TGF)-β induces the differentiation of mesenchymal cells into a chondrocyte phenotype and stimulates bone formation.5, 6, 7 BMP and TGF-β are related with the development of ossification of the PLL in OPLL.8 Proliferating cell nuclear antigen (PCNA) functions as a cofactor for DNA polymerases in DNA synthesis and is also cell proliferation marker.9, 10 Along with this, alkaline phosphatase (ALP), a marker of terminal differentiation of chondrocytes, is involved in cartilage matrix calcification.11 Finally, osteopontin (OPN) is a major extracellular matrix component in bone and is primarily made by cells of the osteoblastic lineage, but is also synthesized by chondrocytes in the area of the cartilage-to-bone transition.12, 13
To better determine whether HPLL progresses to OPLL, factors including BMP, TGF-β, PCNA, ALP and OPN were studied in eight specimens obtained from myelopathic patients with HPLL and two with OPLL. Results were compared with those obtained from comparable analyses of the PLL from two control subjects with spondylosis alone.
Materials and methods
Subjects
During microscopic anterior decompressive surgery, eight specimens of HPLL and two OPLL were resected for decompression of the spinal cord. Two cases were prepared from cervical spondylosis as controls, which were normal in shape and thickness. In HPLL, there were six men and two women with ages ranging from 50–84 years with a mean of 64.6 years (Table 1).
Materials
The specimens were fixed in 10% neutral buffered formalin. After washing with 0.01 ml/l pH 7.2 phosphate-buffered saline (PBS), they were decalcified with formic acid for 7 days. They were then placed in sodium nitrate for 1 h for the neutralization of formic acid and washed by water to remove the formic acid and sodium nitrate. The specimens were next embedded in paraffin and sliced into 4 μm-thick sections, which were then subjected to hematoxylin and eosin (H&E) and alcian blue staining and immunohistochemical staining using antibodies specific for BMP-2/4, TGF-β and PCNA.
Immunostaining
Immunostaining was performed by the avidin–biotin–peroxidase complex methods. A kit was used for immunostaining with goat polyclonal antibodies against BMP-2/4 (R&D Systems, MN, USA), mouse monoclonal antibody against TGF-β (R&D Systems, MN, USA), rat monoclonal antibody against PCNA (ICN pharmaceuticals, OH, USA), mouse monoclonal antibody against ALP (R&D Systems, MN, USA) and mouse monoclonal antibody against OPN (IBL, Gunma, Japan). Endogenous peroxidase was blocked with methanol containing 3% hydrogen peroxide. Specimens were incubated with PBS containing 1% bovine serum albumin for 30 min at room temperature to avoid nonspecific protein binding and then with the appropriate concentration of primary antibodies at 4°C overnight in a humidified chamber. After washing with PBS three times for 10 min, specimens were reacted with biotinylated secondary antibody for 30 min and incubated with streptavidin–biotin–peroxidase complex/horseradish peroxidase (Dako, Glostrup, Denmark) in a humidified chamber for 30 min at room temperature. The specimens were washed with PBS after each step. Color was developed using 3,3′-diaminobenzidine tetrahydrochloride. Also, sections were counterstained by hematoxylin for staining of their nuclei. As negative controls, normal rabbit immunoglobulin G, normal mouse immunoglobulin G and antibodies were used instead of the primary antibodies.
Results
Histological findings
Histological characteristics of HPLL
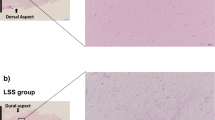
On H&E staining, hyalinoid degeneration, the proliferation of fibroblast-like spindle cells and chondrocytes, infiltration of vessels, distorted and disrupted ligament fibers, different degrees of staining with alcian blue and small ossification were observed (Figures 1 and 2).
H&E staining of HPLL. (a) In four cases (Cases 5–8 in Table 2), chondroid tissue was prominent. Ligament fibers were replaced by cartilaginous tissues. (b) Most cells were chondrocytes. (c) In some areas (magnification of arrow in a), fibroblasts (arrowhead) and fibroblast-like spindle cells (arrow) were observed. Fibrous tissues were relatively well preserved. (d) Chondroid tissue was strongly stained by alcian blue. Magnification, (a) × 10, (b–d); × 40
OPLL
In OPLL, the histological findings were comparable in all cases. Fibroblast and fibroblast-like spindle cells were found at sites remote from the ossified mass, where the collagen fibers were rough and loose. Chondrocytes or hypertrophic chondrocytes were present in calcified areas near the ossified mass.
PLL
In the control cases, normal PLL showed relatively well-aligned fibrous tissue with flattened fibroblasts. No ossification or cartilaginous tissue was found.
Immunostaining by BMP, TGF-β and PCNA
HPLL
Fibroblast-like spindle cells were moderately stained in four cases by BMP and in four cases by TGF-β. PCNA was moderately or weakly detected in all fibroblast-like spindle cells (Figure 3). Chondrocytes showed moderate expression with BMP, TGF-β and PCNA and showed enhanced expression, especially in the cartilage prominent area, compared to fibroblast-like spindle cells (Figure 4). In two cases, which were combined with disc herniation, fibroblast-like spindle cells and chondrocytes showed more enhanced expression by BMP, TGF-β and PCNA than did the other cases. Fibroblasts in the fibrous area expressed by BMP, TGF-β and PCNA, but those along the margin of HPLL did not. The immunohistochemical findings of HPLL are summarized in Table 2.
OPLL
In OPLL, chondrocytes in and around the calcified cartilage area showed enhanced expression of BMP and TGF-β compared to fibroblast and fibroblast-like spindle cells in the fibrous tissue remote from the ossified mass. Staining by PCNA showed similar results for BMP and TGF-β.
PLL
In the PLL cells of the control cases, no staining by BMP, TGF-β and PCNA was observed.
Immunostaining by APL and OPN
In four cases of HPLL with prominent chondroid tissue, chondrocytes were expressed by ALP and OPN (Figure 5). Fibroblast-like spindle cells showed no staining. Also, control PLL cells showed no expression by ALP and OPN. In OPLL, chondrocytes near the carilage area and osteocytes in the ossified mass expressed ALP and OPN.
Discussion
Histological characteristics of HPLL
The histological findings of HPLL, proliferation of fibroblast-like spindle cells and chondrocytes, infiltration of vessels and small ossification were compatible with those of previous reports.14, 15, 16 Fibroblast-like spindle cells are a transitional type of cells during the differentiation of fibroblasts into chondrocytes or osteogenic cells.8, 17, 18 As HPLL advances, chondrocytes and ligament fibers become increasingly replaced by cartilaginous tissue.
Immunohistochemical findings of HPLL
PCNA expressed in HPLL but not control subjects, reflected the acceleration of cell growth associated with HPLL. Positive expression of fibroblast-like spindle cells and chondrocytes of BMP, TGF-β in HPLL cells, and their similarity to OPLL suggest that these cells have the potential for transforming into OPLL. Chondrocytes, especially in the cartilage prominent area, showed enhanced expression of BMP, TGF-β and PCNA, suggesting that the upregulation may play a role in their differentiation of cells into osteogenic cells as well as ligamentous hypertrophy. These cells expressed ALP and OPN, so this area seemed to be calcified cartilaginous tissue.19 This therefore suggests that this cartilage may be replaced by new bone.
The relationship between HPLL and OPLL
Two theories have emerged regarding the relationship between HPLL and OPLL. The first is that there is no relationship between two; the other is that HPLL is a precursor to OPLL.1, 2, 15, 16, 20 The histological and immunohistochemical results of this study support the latter hypothesis that HPLL transforms into or constitutes an earlier stage of OPLL.
References
Mizuno J, Nakgawa H, Hashizume Y . Analysis of hypertrophy of the posterior longitudinal ligament of the cervical spine, on the basis of clinical and experimental studies. Neurosurgery 2001; 49: 1091–1098.
Motegi H et al. Proliferating cell nuclear antigen in hypertrophied spinal ligaments. Spine 1998; 23: 305–310.
Urist MR, DeLange RJ, Finerman GA . Bone cell differentiation and growth factors. Science 1983; 220: 680–686.
Wang EA et al. Recombinant human bone morphogenetic protein induces bone formation. Proc Natl Acad Sci USA 1990; 87: 2220–2224.
Carrington JL . Accumulation, localization, and compartmentation of transformation growth factor-β during endochondral bone development. J Cell Biol 1988; 107: 1969–1975.
Joyce ME, Roberts AB, Sporn MB, Bolander ME . Transforming growth factor-β and initiation of chondrogenesis and osteogenesis in the rat femur. J Cell Biol 1990; 110: 2195–2207.
Noda M, Camilliere JJ . In vivo stimulation of bone formation by transforming growth factor-β. Endocrinology 1989; 124: 2991–2994.
Kawaguchi H et al. Immunohistochemical demonstration of bone morphogenic protein-2 and transforming growth factor-β in the ossification of the posterior longitudinal ligament of the cervical spine. Spine 1992; 17: S33–S36.
Hall PA et al. Proliferating cell nuclear antigen (PCNA) immunolocalization in paraffin sections: an index of cell proliferation with evidence of deregulated expression in some neoplasms. J Pathol 1990; 162: 285–294.
Waseem NH, Lane DP . Monoclonal antibody analysis of the proliferating cell nuclear antigen (PCNA). Structural conservation and the detection of a nucleolar form. J cell Sci 1990; 96: 121–129.
Kato Y et al. Terminal differentiation and calcification in rabbit chondrocyte cultures grown in centrifuge tubes: regulation by transforming growth factor beta and serum factors. Proc Natl Acad Sci USA 1988; 85: 9552–9556.
Franzen A, Oldberg A, Solursh M . Possible recruitment of osteoblastic precusor cells from hypertrophic chondrocytes during initial osteogenesis in cartilaginous limbs of young rats. Matrix 1989; 9: 261–265.
McKee MD et al. Developmental appearance and ultrastructural immunolocalization of a major 66 kDa phosphoprotein in embryonic and post-natal chicken bone. Anat Rec 1990; 228: 77–92.
Kamikozuru M, Yamaura I . Hypertrophy of the cervical posterior longitudinal ligament (a provisional name) causing myelopathy: a case report. Presented at the second annual meeting of the Japan Spine Research Society Tokyo, Japan, November 9, 1974 (in Japanese).
Kurata A et al. Myelopathy caused by hypertrophy of the posterior longitudinal ligament (HPLL): Case report. Noushinkeigeka 1987; 15: 651–655 (Abstract in English).
Toguchi A et al. Myelopathy due to hypertrophy of the posterior longitudinal ligament in cervical spine: an operative case. Kanto J Orthopaed Traumatol 1992; 23: 172–177 (in Japanese).
Hoshi K et al. Fibroblasts of spinal ligaments pathologically differentiate into chondrocytes induced by recombinant human bone morphogenetic protein-2: morphological examinations for ossification of spinal ligaments. Bone 1997; 21: 155–162.
Yonemori K et al. Bone morphogenetic protein receptors and activin receptors are highly expressed in ossified ligaments tissues of patients with ossification of the posterior longitudinal ligament. Am J Pathol 1997; 150: 1335–1347.
Lee K et al. Parathyroid hormone induces sequential c-fos expression in bone cells in vivo: in situ localization of its receptor and c-fos messenger ribonucleic acids. Endocrinology 1994; 134: 441–450.
Yamazaki A, Homma T, Ishikawa S, Okumura H . Magnetic resonance imaging and histologic study of hypertrophic cervical posterior longitudinal ligament. A case report. Spine 1991; 16: 1262–1266.
Acknowledgements
We thank K Kotani and T Mizuno for their technical assistance.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Song, J., Mizuno, J., Hashizume, Y. et al. Immunohistochemistry of symptomatic hypertrophy of the posterior longitudinal ligament with special reference to ligamentous ossification. Spinal Cord 44, 576–581 (2006). https://doi.org/10.1038/sj.sc.3101881
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101881
Keywords
This article is cited by
-
Expression of proinflammatory cytokines and proinsulin by bone marrow-derived cells for fracture healing in long-term diabetic mice
BMC Musculoskeletal Disorders (2023)
-
Cyclic tensile strain facilitates ossification of the cervical posterior longitudinal ligament via increased Indian hedgehog signaling
Scientific Reports (2020)
-
Ossification of the posterior longitudinal ligament in the cervical spine: a review
International Orthopaedics (2019)
-
Ossification process involving the human thoracic ligamentum flavum: role of transcription factors
Arthritis Research & Therapy (2011)
-
High glucose promotes collagen synthesis by cultured cells from rat cervical posterior longitudinal ligament via transforming growth factor-β1
European Spine Journal (2008)