Abstract
Study design: Investigation of five patients receiving an implant, using laboratory cystometry and self-catheterisation at home.
Objectives: To use the established Finetech-Brindley sacral root stimulator to increase bladder capacity by neuromodulation, eliminating the need for posterior rhizotomy, as well as achieving bladder emptying by neurostimulation.
Setting: Spinal Injuries Unit, Royal National Orthopaedic Hospital, Stanmore, Middlesex, UK.
Methods: Five patients underwent implantation of a Finetech-Brindley stimulator without rhizotomy of the posterior roots. This was either a two channel extradural device (four cases) or a three channel intrathecal device (one case). In each patient, the implant was configured as a Sacral Posterior and Anterior Root Stimulator (SPARS). Postoperatively, repeated provocations using rapid instillation of 60 ml saline were used to determine the relative thresholds for neuromodulation using each channel. The effect of continuous neuromodulation was examined in the laboratory using slow fill cystometrograms, and conditional stimulation was also studied (neuromodulation for 1 min to suppress hyperreflexic contractions as they occurred). In one patient, neuromodulation was applied continuously at home, and volumes at self catheterisation recorded in a diary.
Results: Reflex erections were preserved in each patient. In three patients, detrusor hyperreflexia persisted postoperatively and neuromodulation via the implant was studied. In these three patients, the configuration was: S2 mixed roots bilaterally (channel B), and S34 bilaterally (channel A). Both channels could be used to suppress provoked hyperreflexic contractions, with the S2 channel effective at a shorter pulse width than S34 in a majority of cases. Continuous stimulation more than doubled bladder capacity in two out of three patients during slow fill cystometry. Conditional stimulation was highly effective. In the one patient who used continuous stimulation at home, bladder capacity was more than doubled and the effect was comparable with anticholinergic medication. Bladder pressures >70 cm water could be achieved with intense stimulation in three patients, but detrusor-external urethral sphincter dyssynergia (DSD) prevented complete emptying.
Conclusions: Neuromodulation via a SPARS was effective and may replace the need for posterior rhizotomy. However, persisting DSD may prevent complete bladder emptying and warrants further investigation.
Similar content being viewed by others
Introduction
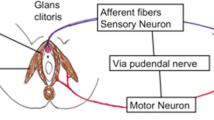
It has been known for some time that stimulation of the pudendal afferents or sacral nerve roots suppresses bladder activity. This effect is observed in normal subjects,1 idiopathic bladder instability,2 and in the detrusor hyperreflexia that is the likely consequence of spinal cord injury.3 It can be termed neuromodulation, where ‘the influence of activity in one neural pathway affects the pre-existing activity in another by synaptic interaction.4
Untreated detrusor hyperreflexia may cause incontinence – and when it occurs with detrusor-external sphincter dyssynergia, high bladder pressures and, if untreated, vesicoureteric reflux leading to renal impairment. Preventing this sequence of events is a primary aim of bladder management in those with spinal cord injury.
Initial experiments with anal stimulation5 and penile squeeze6 demonstrated bladder suppression in SCI patients, and over the last 20 years four centres have shown that skin stimulation of the Dorsal Penile Nerve can reliably increase bladder capacity.3,7,8,9 Stimulation of the sacral roots, whether magnetically10 or by percutaneous11 or implanted electrode,12 is probably at least as effective. However, although there is now a large quantity of data about the short term effects of neuromodulation, it has been more difficult to apply it in the long term. Two groups have shown that application of sacral root stimulation via implanted electrodes can increase capacity to a functionally useful degree,12,13 but the situation in spinal cord injury lags behind the treatment of the urinary urge group, where the Medtronic Interstim is an accepted and effective implant.2
The Finetech-Brindley Sacral Anterior Root Stimulator (SARS or Vocare, Neurocontrol, USA) is an established and successful device for bladder and bowel emptying in Spinal Cord Injury, and is usually accompanied by a rhizotomy of the posterior (sensory) sacral roots. Although the early SARS devices were often implanted without rhizotomy, the procedure became standard as its great benefits were realised: a low pressure, high capacity bladder and elimination of active detrusor-external urethral sphincter dyssynergia.14 However, rhizotomy is unacceptable to many patients because it abolishes reflex erection and ejaculation, and is destructive – the latter becoming an increasingly important factor as the prospects for spinal cord regeneration improve. It may also cause sphincter and pelvic floor weakness, and in a minority of patients, stress incontinence.15
Without rhizotomy, a SARS device has the potential to be used for neuromodulation to increase bladder capacity, and neurostimulation for bladder and bowel emptying. It is then a Sacral Posterior and Anterior Root Stimulator – SPARS. Because the effects of neuromodulation are mediated by myelinated A afferent fibres,16 it can be achieved by low-level stimulation of the mixed sacral roots, with more intense stimulation to activate preganglionic efferent B fibres to empty the bladder and bowel when required. Use of an extradural device to stimulate the mixed nerves is simpler and probably safer than intradural separation into anterior and posterior roots.
The aim of this study was to establish the efficacy of both acute and chronic neuromodulation via a Finetech-Brindley stimulator in SPARS configuration. We describe three patients in which neuromodulation and bladder stimulation have been achieved with this device.
Materials and Methods
Local ethics committee approval and informed consent were obtained. Five patients were implanted with SPARS devices. All had detrusor hyperreflexia (as defined by International Continence Society17) preoperatively, but in two patients this was not present postoperatively. The patients are listed in Table 1.
Cystometry
Anticholinergic medication was stopped at least 4 days before cystometry. In all tests, a filling rate of 10 ml/min was used. This was chosen to be as close as possible to natural filling while allowing a sufficient number of cystometrograms to be performed on one day. Filling was stopped when there was a sustained rise in bladder pressure of >35 cm water, or incontinence – ‘firing off’. Bladder capacity was calculated by adding the volume fired off to the residual measured by aspiration. Two types of urethral catheter were used: either standard urodynamics catheters (10 French filling and small bore pressure catheter) or a four channel microtip pressure transducer (Gaeltech, Isle of Skye, UK). The latter has three urethral and one bladder pressure transducer; between them is an asymmetric balloon which, with gentle pulling, lodges in the bladder neck. The position of the catheter was confirmed by observing rapid, large pressure rises with SPARS stimulation at the urethral transducers, characteristic of external urethral sphincter contraction. All tests were conducted in the supine position.
Preoperative tests
In each patient, two control cystometrograms were performed to establish baseline bladder capacity. The effects of neuromodulation via Dorsal Penile Nerve stimulation were then studied. Stimulation was via Ag/AgCl self-adhesive electrodes, at a frequency of 15 Hz, pulse width 200 μs and current set at twice the threshold for the pudendo-anal reflex. The parameters used were derived from previous work at our institution using provoked contractions.18 Bladder capacity with continuous stimulation was measured during three fills with neuromodulation. If possible, a final control fill was performed to assess the residual effects of neuromodulation.
Implantation
In four patients (AS, GD, DL, PG), a laminectomy from L5 to S2 was performed. Standard Finetech-Brindley extradural electrodes (Neurocontrol, Cleveland, USA) were placed bilaterally on the mixed S2 roots (channel B) and bilaterally on the mixed S3 and S4 roots (channel A). In one patient (SN), a three channel intrathecal implant was used, with electrodes placed bilaterally on S3 anterior roots (channel A), S3 posterior roots (channel B) and S4 mixed roots (channel C).
Postoperative tests
Postoperative cystometry was used to confirm hyperreflexia and record baseline bladder capacity. The effects of sacral root stimulation were then examined in detail, as follows:
Provocations
Repeated rapid instillations of 60 ml Normal Saline over 5–10 s at room temperature were used to provoke hyperreflexic contractions. Provocation was deemed successful if detrusor pressure rose by 15 cm water or more. In almost all cases such a pressure rise indicated the start of a hyperreflexic contraction. If the provocation was not successful, a further 60 ml was instilled. Control and neuromodulation provocations were interleaved, and stimulation was always conditional – applied only once the bladder pressure rise of 15 cm water had occurred. At the end of each provocation test, 60 ml was aspirated from the bladder.
As in the tests with Dorsal Penile Nerve stimulation, frequency was set at 15 Hz. The Finetech-Brindley device allows only large variations in the intensity of stimulation, and for neuromodulation this was always set at 1 (the lowest available). The pulse width was varied from 8 to 256 μs to determine the threshold value for successful neuromodulation. This also allowed determination of thresholds for urethral and anal sphincter contraction.
Slow fills
After two control cystometrograms, bladder capacity was measured with continuous stimulation via the SPARS. The pulse width was set at between 1.5 and 5 times the threshold level determined using provoked contractions for both channels. This was always several times less than the level necessary to produce a bladder contraction. In addition, in one patient alternating fills were performed using the S2 and S34 channels to confirm the efficacy of neuromodulation with each.
In two patients, the effects of conditional neuromodulation were studied. Here, stimulation was applied for 1 min at the start of a hyperreflexic contraction9–defined as a rise in intravesical pressure of greater than 10 cm water. If the contraction was not completely suppressed after 1 min, stimulation was continued for a further minute. These parameters were derived from previous work on conditional neuromodulation via the dorsal penile nerve.9 The criteria for ending filling were the same as during continuous fills.
Long term neuromodulation
In one patient, the transmitter of the Finetech-Brindley device was fixed to the skin using a variety of stoma management materials, and continuous stimulation applied at between three and six times the threshold for suppression of provoked contractions. Two identically programmed transmitter boxes were used alternately, to allow charging. Bladder capacity was measured by self catheterisation, and where incontinence occurred before catheterisation, an attempt was made to estimate its volume. A program of 50 s on, 50 s off stimulation was tried and comparison periods with no stimulation and with oxybutinin were included.
Bladder emptying
Conventional interval voiding programs were tried in each patient, and optimised using videourodynamics and the multitip urethral pressure transducer.
Erectile function
Patients completed a questionnaire of erectile function before and after the implant. Because reflex erections in spinal cord injury are often variable, we considered this more accurate than testing erectile function in the laboratory.
Criteria for measurement and data analysis
In the preoperative and postoperative tests using slow filling, bladder volume at firing off or sustained detrusor pressure rise >35 cm water was recorded for each fill.
In the postoperative experiments using provoked contractions, the primary measure was the threshold pulse width for successful neuromodulation using each channel. This was derived by plotting the area under 60 s of the bladder pressure-time trace for each provocation, with the data fitted where possible to a Boltzmann sigmoid curve. If the fit was not possible, a best-fit line was drawn manually. The threshold for neuromodulation was defined as the pulse width which gave 50% of maximum suppression. For each patient, the provocation tests were repeated on a separate day and the mean of the two thresholds calculated (a total of 6 days' testing).
In the home experiments, bladder volume at self catheterisation was the primary measure. Results were compared using a two-tailed Mann-Whitney U test with a significance level of 95%.
Results
Preoperative neuromodulation with dorsal penile nerve stimulation
This resulted in at least a 70% increase in bladder capacity in four patients (GD, DL, SN, PG). In AS, detrusor hyperreflexia occurred at a bladder volume of 400–500 ml, so that continuous neuromodulation was not tried; previous experiments had demonstrated suppression of provoked hyperreflexic contractions with dorsal penile nerve stimulation. The results are shown in Figure 1. Volume increased progressively with each neuromodulation fill.
Postoperative findings
In all five patients, reflex erections were preserved. In four patients, they were ‘no different’ compared to preoperatively, and in AS (who had other evidence of damage to the posterior roots) they were still present but less frequent than before implantation.
In two patients (AS and SN), detrusor hyperreflexia was present preoperatively but was not reproducible postoperatively either during slow filling or on provocation with rapid instillation of saline. In both cases, changes in the threshold for the DPN reflex and skeletal muscle and bladder responses to stimulation suggested partial sacral root damage. These patients will not be described in the sections on postoperative neuromodulation that follow.
Postoperative tests using provoked contractions
In each of the three patients, it was possible to determine thresholds for suppression of provoked contractions using each channel of the stimulator. A sample series of provocations (with controls) is shown in Figure 2, and the method for calculation in Figure 3.
There was considerable variation between days 1 and 2 in the calculated thresholds for neuromodulation (Table 2). In four out of six tests, the threshold pulse width for suppression of provoked contractions was lower with the S2 channel, and the mean threshold for the 2 days testing was lower with S2 in two out of three patients.
The threshold for neuromodulation was also expressed as a multiple of the threshold for urethral or anal sphincter contraction. adjusted in this way, the mean S2 threshold is lower than the mean S34 threshold in each patient (Table 2). Anal and urethral sphincter pressure rises at the threshold for neuromodulation were not consistently higher with S2 or S34.
The degree of skeletal muscle contraction at the threshold for neuromodulation using each channel is shown in Table 3.
Slow fill cystometry with neuromodulation
In each patient, neuromodulation increased bladder capacity. DL has high pressure detrusor hyperreflexia, and neuromodulation on three occasions has increased his capacity by no more than 35%. Results in PG and GD are better (Figure 4). Conditional neuromodulation via SPARS was also very effective (Figures 5, 6).
Long term neuromodulation
In one subject (PG), neuromodulation was used intermittently at home for a period of 4 months. The volumes at self-catheterisation are shown in Figure 7. The patient found it difficult to wear a sheath, and used an indwelling catheter when urine output was likely to be high or catheterisation inconvenient. This also made accurate estimation of the volume fired off difficult.
The median volume at self-catheterisation (excluding volume fired off) was 100 ml in the control period, and 250 ml in the continuous, on/off and oxybutinin periods. The difference between control and all other periods was highly significant (P<0.0001) in each case. There was not a significant difference between continuous, on/off and oxybutinin periods.
Bladder emptying
In one patient (AS), complete bladder emptying was achieved with intense intermittent stimulation of the S34 channel. However, this patient had mild detrusor hyperreflexia and little evidence of detrusor-sphincter dyssynergia preoperatively. After implantation, neither was present, so that the behaviour of his bladder was similar to patients who have undergone posterior rhizotomy.
In three patients (DL, PG and SN), intense stimulation resulted in detrusor pressure >70 cm water. Each had incomplete (less than 50%) bladder emptying, and clear evidence of dyssynergic external urethral sphincter contractions (and periurethral and pelvic floor muscle contraction in PG) in the gaps between bursts of stimulation. Examples are shown in Figure 8.
In one patient (GD) it is not yet possible to achieve significant detrusor pressure with stimulation, in spite of intact hyperreflexia and good bladder pressure rises during intraoperative stimulation. The failure to produce a bladder contraction is probably due to a combination of electrode malposition and neuropraxia.
Discussion
At the start of this study, we tested the response to Dorsal Penile Nerve stimulation to ensure that patients responded to neuromodulation before embarking on a SPARS implant. In our experience, bladder capacity almost always increases9 with this method of neuromodulation if the intensity of stimulation is set at an optimum level. Testing neuromodulation by percutaneous sacral nerve stimulation (in a similar way to the Peripheral Nerve Evaluation (PNE) test used before implantation of the Medtronic Interstim11) is more invasive and in this case may not necessarily be more informative: if a patient responds to dorsal penile nerve stimulation, it is reasonable to suppose that the same afferent fibres can be activated by an implant capable of stimulating sacral roots 2 to 4.
In the four patients tested, dorsal penile nerve stimulation markedly increased bladder capacity. The results of laboratory tests are probably best in patients with significant hyperreflexia who have good bladder capacities with anticholinergics: the capacity falls rapidly on stopping this medication, and is restored with neuromodulation. For this reason, the results with acute neuromodulation using different techniques should be compared with caution: the improvement in bladder capacity is highly dependent on the patient group.
This variability means that any attempt to compare the efficacy of neuromodulation via different sacral roots should either involve large numbers of patients, or a method where different roots may be stimulated in the same patient. Although our series consisted of only three patients, it presented a valuable opportunity for the latter. Provocation of unstable contractions is quick and reproducible, allowing the evaluation of a large number of parameter changes during 1 day of testing.
The stimulation program in the Finetech-Brindley control box allows fine variation in pulse width rather than amplitude. At the settings used here, charge delivered is proportional to pulse width and therefore approximates to intensity, but is not exactly equivalent.19
Consistent with previous findings,10,20 detrusor hyperreflexia could be reliably provoked many times in each patient, with a gap of only 3 min between episodes of neuromodulation. That the residual effect of neuromodulation after several minutes was not sufficient to prevent a hyperreflexic contraction contrasts with the persistent effects seen during repeated slow fills with continuous neuromodulation, where the effects on bladder volume at first contraction seem to persist for several hours.9 The likely explanation is that the inhibition of the detrusor reflex gradually diminishes: after 3 min it can be overcome by an intense stimulus (such as the provocation described in this study), but a smaller inhibition can influence the threshold for detrusor hyperreflexia during slow bladder filling. The aim of interleaving of provocations with and without neuromodulation during this study was to minimise the influence of such carry over effects. In all three patients, we found that the area under the detrusor pressure curve for control provocations was not markedly affected by preceding neuromodulation.
Although the results of each day's testing allowed calculation of threshold pulse widths, agreement between the 2 days was poor in DL and only fair in PG. DL has never had marked increases in bladder capacity with neuromodulation, implying that the effect of sacral root stimulation is weak in him, and this may be reflected in the variability of the results with provocation. In PG and GD, S2 stimulation produced neuromodulation at a lower (three out of four tests) or similar (one test) threshold compared to S34, and normalisation of the neuromodulation threshold to the threshold for anal and urethral sphincter contraction did not markedly affect the findings. In GD, however, where the difference is largest, there is certainly a degree of neuropraxia of the S34 roots, because bladder contractions cannot be achieved. It is therefore likely that there is some neuropraxia of the S34 fibres responsible for neuromodulation, which may account for some of the difference between S2 and S34.
Schmidt has asserted that ‘The key to control of the bladder lies in control of the sphincter,21 and has also observed that stimulation of S2 gives larger external urethral sphincter contractions than S3 or S4,22 which is consistent with our finding that S2 was in most cases effective at a lower pulse width than S34. However, there is not agreement on this point, and others have found that S3 has the largest contribution.23 Also, there are several pieces of evidence suggesting that although sphincter contraction may be a marker for an adequate stimulus for neuromodulation, it is not central to the mechanism. Firstly, skeletal muscle paralysis does not abolish bladder suppression in cats,24 and secondly, stimulation of afferent branches of the pudendal nerve that innervate the region of the external urethral sphincter does not suppress bladder activity in cats, whereas stimulation of afferents from the penis does.25
Although our finding of a generally lower threshold for S2 may be due to several causes (some of them artefactual), we have shown that this root certainly can be used for neuromodulation. It is likely that S3 was chosen for the Interstim device (and for previous trials of long-term stimulation in spinal cord injury) because it produces less skeletal muscle contraction than S2.21 It may be that in neurologically intact patients this does make S3 or S4 preferable, but none of our SCI patients experienced inconvenient skeletal muscle contractions with either S2 or S34 stimulation. Also, one might expect chronic stimulation of the glutei to have a beneficial effect on muscle bulk.
We found that neuromodulation via the SPARS during slow filling can markedly increase bladder capacity: in two patients bladder capacity was more than doubled, and in one it increased by a third. The results were not analyzed statistically because the number of patients was small, bladder capacity is probably not normally distributed and the ‘carry over’ effect from previous stimulations is considerable.
To study the effect of conditional stimulation, the filling rate should be low (50 ml/min is probably too provocative) and it must be possible to turn the stimulation on and off rapidly. These conditions have not been present in previous studies of sacral root neuromodulation.11,12 We used conditional stimulation in the two patients who responded well to continuous stimulation, and found it to be highly effective, consistent with a previous finding that conditional stimulation via the dorsal penile nerve is probably at least as effective as continuous.9 It suggests that current research to develop a device for conditional neuromodulation – capable of detecting bladder pressure rises by recording from the sacral roots, and then suppressing them by stimulation – is justified. The trigger of 10 cm water chosen in this study is similar to the smallest rises in bladder pressure that can be detected by recording from the cat sacral roots.26
In previous studies with implanted stimulators for long-term neuromodulation in SCI12,13 cystometry was performed at predefined intervals after implantation to assess the effect of neuromodulation. Especially with the filling rates used (50 ml/min or greater), this does not necessarily reflect the bladder capacity that the patient experiences at home. It was also not applicable in our case, as the patient used stimulation for variable periods of 4 days to 2 weeks.
Instead, volume at self catheterisation was our primary outcome variable. Recording the volume leaked with a pad and adding this to catheterised volume would have increased the complexity of the measurements and is not necessarily informative: if the patient does not self catheterise immediately after ‘firing off’, bladder capacity will be overestimated. We considered it more reliable (and easier for the patient) to record bladder volumes at self catheterisation for a long period, and to infer the bladder capacity from the maximum volumes achieved without incontinence. Measuring frequency of self-catheterisation is unreliable as the decision to catheterise is a subjective one, and patients may alter their fluid intake according to their bladder management.
Our current method for long term stimulation is not ideal: it is necessary to fix the transmitter coils to the skin over the subcutaneous receiver for all but the first 30–60 min after bladder emptying. However, PG used the device intermittently at home over a period of 4 months, and the results show a marked increase in bladder capacity. When stimulation is stopped, this returns in less than 1 day to a much smaller baseline capacity. The effect of neuromodulation was comparable with oxybutinin, although PG stated that incontinence on filling past bladder capacity is generally of larger volume with neuromodulation – when the bladder ‘escapes’ from electrical suppression, it contracts at close to full force. We did not find that the effect of neuromodulation diminished with time, or that there was a need to increase the stimulation parameters. However, as shown in Figure 8, some incontinence persisted (although stress incontinence was improved with neuromodulation, probably because of persistent urethral sphincter contraction). Volumes with the 50 s on/50 s off program were no worse than with the continuous pattern, and indeed the patient felt strongly that this pattern was more effective than continuous stimulation. He preferred neuromodulation to oxybutinin.
The difficulty in achieving good bladder emptying using the established interval voiding technique is likely to be due to detrusor-external urethral sphincter dyssynergia in DL and SN, and in addition pelvic floor and periurethral muscle spasm in PG. In each case, intact sacral posterior root pathways are likely to be a significant factor. As Brindley suggested,27 bladder emptying would probably be improved by posterior rhizotomy, and we would not currently implant a SPARS device without rhizotomy in patients with severe detrusor-external sphincter dyssynergia. Brindley's early patients often did not have a rhizotomy, and most achieved good emptying, but the devices were intrathecal and there was posterior root damage in many cases.28 We are currently investigating different strategies to improve bladder emptying in the patients described here.
In summary, stimulation of the Dorsal Penile Nerve is a simple and non-invasive screening test for the bladder response to neuromodulation. As well as stimulation for bladder emptying, the Finetech-Brindley device can be used to suppress provoked contractions and markedly increase bladder capacity in the laboratory, and we have shown that it is feasible to use long-term stimulation at home as a replacement for oxybutinin. Conditional neuromodulation of the sacral roots was highly effective and is a promising technique for future implanted devices.
References
Craggs MD et al. Detrusor relaxation following magnetic stimulation of the sacral roots in healthy men J Physiol 1997 501: 53P
Van Kerrebroeck PE . The role of electrical stimulation in voiding dysfunction Eur Urol 1998 34: Suppl 1 27–30
Previnaire JG et al. Short-term effect of pudendal nerve electrical stimulation on detrusor hyperreflexia in spinal cord injury patients: importance of current strength Paraplegia 1996 34: 95–99
Craggs MD, McFarlane JP . Neuromodulation of the lower urinary tract Exp Physiol 1999 84: 149–160
Fossberg E et al. Maximal electrical stimulation in the treatment of unstable detrusor and urge incontinence Eur Urol 1990 18: 120–123
Kondo A, Otano T, Taika T . Suppression of bladder instability by penile squeeze Br J Urol 1982 54: 360–362
Vodusek DB, Light KJ, Libby JM . Detrusor inhibition induced by stimulation of pudendal nerve afferents Neurourol Urodyn 1986 5: 381–389
Wheeler JS, Walter JS, Zaszczurynski PJ . Bladder inhibition by penile nerve stimulation in spinal cord injury patients J Urol 1992 147: 100–103
Kirkham APS et al. The effects of continuous and conditional neuromodulation on the bladder in Spinal Cord Injury Spinal Cord 2001 39: 420–428
Sheriff MK et al. Neuromodulation of detrusor hyper-reflexia by functional magnetic stimulation of the sacral roots Br J Urol 1996 78: 39–46
Chartier-Kastler EJ et al. Urodynamic monitoring during percutaneous sacral nerve neurostimulation in patients with neurogenic detrusor hyperreflexia Neurourol Urodyn 2001 20: 61–71
Ishigooka M et al. A new technique for sacral nerve stimulation: a percutaneous method for urinary incontinence caused by spinal cord injury Br J Urol 1998 81: 315–318
Chartier-Kastler EJ et al. Long-term results of sacral nerve stimulation (S3) for the treatment of neurogenic refractory urge incontinence related to detrusor hyperreflexia J Urol 2000 164: 1476–1480
Koldewijn EL et al. Bladder compliance after posterior rhizotomies and anterior sacral root stimulation J Urol 1994 151: 955–960
Barat M et al. Why does continence fail after Sacral Anterior Root Stimulator? Neurourol Urodyn 1993 12: 507–508
Yalla SV et al. Urethral striated sphincter responses to electro-bulbocavernosus stimulation J Urol 1978 119: 406–409
Abrams P, Blaivas JG, Stanton SL, Anderson JT . Standardisation of terminology of lower urinary tract function Neurourol Urodyn 1988 7: 403–427
Shah N, Knight S, Shah J, Craggs M . Optimisation of stimulation for conditional neuromodulation of detrusor hyperreflexia Eur Urol 1999 35: Suppl 2 16
Bostock H . The strength-duration relationship for excitation of myelinated nerve: computed dependence on membrane parameters J Physiol 1983 341: 59–74
Shah NC, Knight SL, Shah PJR, Craggs MD . Early onset and persistency of neuromodulation for detrusor hyperreflexia in patients with a spinal cord injury Neurourol Urodyn 1998 17: 411–412
Schmidt RA . Advances in genitourinary neurostimulation Neurosurg 1986 18: 1041–1044
Schmidt RA . Applications of neurostimulation in urology Neurourol Urodyn 1988 7: 585–592
Juenemann KP, Lue TF, Schmidt RA, Tanagho EA . Clinical significance of sacral and pudendal nerve anatomy J Urol 1988 139: 74–80
Fall M, Erlanson BE, Carlsson CA, Linström S . The effect of intravaginal electrical stimulation on the feline urethra and urinary bladder. Neuronal Mechanisms Scand J Urol Nephrol 1978 44: Suppl 19–30
Lindström S, Sudsuand R . Functionally specific bladder reflexes from pelvic and pudendal nerve branches: an experimental study in the cat Neurourol Urodyn 1989 8: 392–393
Jezernik S, Grill WM, Sinkjaer T . Detection and inhibition of hyper-reflexive-like bladder contractions in the cat by sacral nerve root recording and electrical stimulation Neurourol Urodyn 2001 20: 215–230
Brindley GS . The first 500 patients with sacral anterior root stimulator implants: general description Paraplegia 1994 32: 795–805
Brindley GS, Polkey CE, Rushton DN, Cardozo L . Sacral anterior root stimulators for bladder control in paraplegia: the first 50 cases J Neurol Neurosurg Psych 1986 49: 1104–1114
Acknowledgements
This work was funded by a clinical fellowship from the Board of Clinical Studies, Royal National Orthopaedic Hospital. Neurocontrol Corporation (Cleveland, Ohio, USA) and Finetech Medical Ltd. (Welwyn Garden City, UK) provided valuable materials and expertise.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kirkham, A., Knight, S., Craggs, M. et al. Neuromodulation through sacral nerve roots 2 to 4 with a Finetech-Brindley sacral posterior and anterior root stimulator. Spinal Cord 40, 272–281 (2002). https://doi.org/10.1038/sj.sc.3101278
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101278
Keywords
This article is cited by
-
Ambulatory urodynamic monitoring assessment of dorsal genital nerve stimulation for suppression of involuntary detrusor contractions following spinal cord injury: a pilot study
Spinal Cord Series and Cases (2020)
-
Design of integrated neural stimulating and recording frontend for bladder control prosthesis
Analog Integrated Circuits and Signal Processing (2017)
-
Long-term follow-up study of outcomes of bladder management in spinal cord injury patients under the care of The Midlands Centre for Spinal Injuries in Oswestry
Spinal Cord (2012)
-
Die Zukunft der invasiven Neuromodulation
Der Urologe (2012)
-
Functional electrical stimulation after spinal cord injury: current use, therapeutic effects and future directions
Spinal Cord (2008)