Abstract
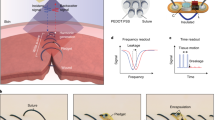
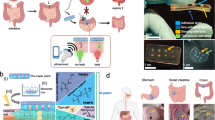
Intestinal wound healing is a long-standing problem, with conventional suturing-based intestinal closure surgeries typically leading to postoperative problems. Alternative strategies or devices capable of effective wound healing have thus been sought. Here we report a self-powered electronic bandage made from soft and biodegradable materials that can accelerate wound healing in the intestine. The device uses dual electrostimulation to promote wound healing: a pulsed electrostimulation that induces electrotransfection of epithelial cells, promoting the expression of healing factors (such as epithelial growth factor); and a d.c. electrostimulation that enhances secretion of healing factors of the transfected cells. The electronic bandage exhibits high transfection efficiency and cell viability for intestinal epithelial cells in vitro, which boosts epithelial growth factor expression during the intraoperative period. Its self-powered galvanic cell from a magnesium and molybdenum microelectrode pair promotes healing factor exocytosis. In vitro and in vivo studies in mice show accelerated intestinal would healing compared to conventional suture-based treatments and an electronic bandage with single electrostimulation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors on reasonable request. Source data are provided with this paper.
References
Schluter, J. et al. The gut microbiota is associated with immune cell dynamics in humans. Nature 588, 303–307 (2020).
Jain, U. et al. Debaryomyces is enriched in Crohn’s disease intestinal tissue and impairs healing in mice. Science 371, 1154–1159 (2021).
David, N. N., Aneel, B., Michael, K. & Douglas, M. B. Stapled versus handsewn intestinal anastomosis in emergency laparotomy: a systemic review and meta-analysis. Surgery 157, 609–618 (2015).
Ho, Y. H. & Ashour, M. A. T. Techniques for colorectal anastomosis. World J. Gastroenterol. 16, 1610–1621 (2010).
Slieker, J. C. et al. Systematic review of the technique of colorectal anastomosis. JAMA Surg. 148, 190–201 (2013).
McElrath, C. et al. Critical role of interferons in gastrointestinal injury repair. Nat. Commun. 12, 2624–2639 (2021).
Wu, J. et al. An off-the-shelf bioadhesive patch for sutureless repair of gastrointestinal defects. Sci. Transl. Med. 14, eabh2857 (2022).
Yuk, H., Zhang, T., Lin, S., Parada, G. A. & Zhao, X. Tough bonding of hydrogels to diverse non-porous surfaces. Nat. Mater. 15, 190–196 (2016).
Batalov, I., Stevens, K. R. & DeForest, C. A. Photopatterned biomolecule immobilization to guide three-dimensional cell fate in natural protein-based hydrogels. Proc. Natl Acad. Sci. USA 118, e2014194118 (2021).
Jaroentomeechai, T. et al. Single-pot glycoprotein biosynthesis using a cell-free transcription-translation system enriched with glycosylation machinery. Nat. Commun. 9, 2686 (2018).
Martino, M. M. et al. Growth factors engineered for super-affinity to the extracellular matrix enhance tissue healing. Science 343, 885–888 (2014).
Yang, Y. et al. Improved pharmacodynamics of epidermal growth factor via microneedles-based self-powered transcutaneous electrical stimulation. Nat. Commun. 13, 6908 (2022).
Shine, A. & Abell, T. L. Role of gastric electrical stimulation in the treatment of gastroparesis. Gastrointest. Disord. 2, 12–26 (2020).
Gutruf, P. et al. Fully implantable optoelectronic systems for battery-free, multimodal operation in neuroscience research. Nat. Electron. 1, 652–660 (2018).
Choi, Y. S. et al. Fully implantable and bioresorbable cardiac pacemakers without leads or batteries. Nat. Biotechnol. 39, 1228–1238 (2021).
Jimbo, H. & Miki, N. Gastric-fluid-utilizing micro battery for micro medical devices. Sens. Actuators B Chem. 134, 219–224 (2008).
Nadeau, P. et al. Prolonged energy harvesting for ingestible devices. Nat. Biomed. Eng. 1, 0022 (2017).
Mostafalu, P. & Sonkusale, S. Flexible and transparent gastric battery: energy harvesting from gastric acid for endoscopy application. Biosens. Bioelectron. 54, 292–296 (2014).
Tsang, M. et al. Biodegradable magnesium/iron batteries with polycaprolactone encapsulation: a microfabricated power source for transient implantable devices. Microsyst. Nanoeng. 1, 15024–15023 (2015).
Kim, Y. J. et al. Biologically derived melanin electrodes in aqueous sodium-ion energy storage devices. Proc. Natl Acad. Sci. USA 110, 20912–20917 (2013).
Yin, L. et al. Materials, designs, and operational characteristics for fully biodegradable primary batteries. Adv. Mater. 26, 3879–3784 (2014).
Wang, L. et al. A fully biodegradable and self-electrified device for neuroregenerative medicine. Sci. Adv. 6, eabc6686 (2020).
Hamedi, H. et al. Chitosan based bioadhesives for biomedical applications: a review. Carbohydr. Polym. 282, 119100–119111 (2022).
Mati-Baouche, N. et al. Chitosan as an adhesive. Eur. Polym. J. 60, 192–212 (2014).
Ren, Z. et al. Rapid self-healing and self-adhesive chitosan-based hydrogels by host-guest interaction and dynamic covalent bond as flexible sensor. Carbohydr. Polym. 273, 118533–118544 (2021).
Suo, H. et al. Interpenetrating polymer network hydrogels composed of chitosan and photocrosslinkable gelatin with enhanced mechanical properties for tissue engineering. Mater. Sci. Eng. C. 92, 612–620 (2018).
Yuk, H. et al. Skin-inspired hydrogel-elastomer hybrids with robust interfaces and functional microstructures. Nat. Commun. 7, 12028–12038 (2016).
Yuk, H., Wu, J. & Zhao, X. Hydrogel interfaces for merging humans and machines. Nat. Rev. Mater. 7, 935–952 (2022).
Healy, K. E. & Ducheyne, P. The mechanisms of passive dissolution of titanium in a model physiological environment. J. Biomed. Mater. Res. 26, 319–338 (1992).
Hussain, H. et al. Structure of a model TiO2 photocatalytic interface. Nat. Mater. 16, 461–466 (2017).
Choi, J. J. E. et al. Mechanical properties of human oral mucosa tissues are site dependent: a combined biomechanical, histological and ultrastructural approach. Clin. Exp. Dent. Res. 6, 602–611 (2020).
Liu, J., Qu, S., Suo, Z. & Yang, W. Functional hydrogel coatings. Natl Sci. Rev. 8, nwaa254 (2021).
Mann, G. E., Sumitomo, T., Cáceres, C. H. & Griffiths, J. R. Reversible plastic strain during cyclic loading-unloading of Mg and Mg-Zn alloys. Mater. Sci. Eng. A 456, 138–146 (2007).
Chen, X. et al. Achieving ultra-high ductility and fracture toughness in molybdenum via Mo2TiC2 MXene addition. Mater. Sci. Eng. A 818, 141422 (2021).
Gallego-Perez, D. et al. Topical tissue nano-transfection mediates non-viral stroma reprogramming and rescue. Nat. Nanotechnol. 12, 974–979 (2017).
Pang, S., Gao, Y. & Choi, S. Flexible and stretchable biobatteries: monolithic integration of membrane-free microbial fuel cells in a single textile layer. Adv. Energy Mater. 8, 1702261 (2018).
Zhao, X. et al. Photocrosslinkable gelatin hydrogel for epidermal tissue engineering. Adv. Healthc. Mater. 5, 108–118 (2016).
Leppik, L., Oliveira, K. M. C., Bhavsar, M. B. & Barker, J. H. Electrical stimulation in bone tissue engineering treatments. Eur. J. Trauma Emerg. S. 46, 231–244 (2020).
Sigismund, S., Lanzetti, L., Scita, G. & Di Fiore, P. P. Endocytosis in the context-dependent regulation of individual and collective cell properties. Nat. Rev. Mol. Cell Biol. 22, 625–643 (2021).
Cheng, L. & Hill, A. F. Therapeutically harnessing extracellular vesicles. Nat. Rev. Drug Discov. 21, 379–399 (2022).
Starling, S. Probing α-cell dysfunction in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 18, 195 (2022).
Racioppi, L. et al. CaMKK2 in myeloid cells is a key regulator of the immune-suppressive microenvironment in breast cancer. Nat. Commun. 10, 2450 (2019).
Stewart, L. M. et al. CaMKK2 facilitates golgi-associated vesicle trafficking to sustain cancer cell proliferation. Cell Death Dis. 12, 1040 (2021).
Choi, Y. S. et al. Stretchable, dynamic covalent polymers for soft, long-lived bioresorbable electronic stimulators designed to facilitate neuromuscular regeneration. Nat. Commun. 11, 5990 (2020).
Rickard, J. et al. Surgical infections in low- and middle-income countries: a global assessment of the burden and management needs. Surg. Infect. 21, 478–494 (2020).
Sommer, F., Anderson, J. M., Bharti, R., Raes, J. & Rosenstiel, P. The resilience of the intestinal microbiota influences health and disease. Nat. Rev. Microbiol. 15, 630–638 (2017).
Yao, G. et al. A self-powered implantable and bioresorbable electrostimulation device for biofeedback bone fracture healing. Proc. Natl Acad. Sci. USA 118, e2100772118 (2021).
Song, J. W. et al. Bioresorbable, wireless, and battery-free system for electrotherapy and impedance sensing at wound sites. Sci. Adv. 9, eade4687 (2023).
Huang, Y. et al. Implantable electronic medicine enabled by bioresorbable microneedles for wireless electrotherapy and drug delivery. Nano Lett. 22, 5944–5953 (2022).
Jiang, Y. et al. Wireless, closed-loop, smart bandage with integrated sensors and stimulators for advanced wound care and accelerated healing. Nat. Biotechnol. 41, 652–662 (2023).
Acknowledgements
This work was supported by National Key Research and Development Program of China (grant nos. 2022YFB3205600 and 2023YFC2415900), National Natural Science Foundation of China (grant nos. 32071407, 62003023), Beijing Natural Science Foundation (grant no. 7212204) (all to L.C.); and National Natural Science Foundation of China (grant no. 32101088) and Beijing Nova Program (grant nos. Z2111000021211133, 20220484225) (all to L.W.). We acknowledge critical support and infrastructure provided for this work by the Feng Chen. Zhejiang University of Technology, School of Materials Science and Engineering, and we thank M. Li, M. Yang and Ms. L. Du for their assistance.
Author information
Authors and Affiliations
Contributions
L.C., C.Y., L.W. and Y.F. supervised the project. L.C., H.L., H.W., Yuqiong Wang and L.W. designed the E-bandage (ET) system and experiment. H.W. conducted the in vitro experiments. Yuqiong Wang discovered the role of Ca2+ influx in EVs secretion. Yuqiong Wang and Y.H. conducted the in vivo mice experiments. H.W., H.L., Yuqiong Wang, X.J., Youdi Liu, X.Y., L.W., C.Y. and L.C. prepared the manuscript. H.W., H.L., Yi Wang, T.C. and L.C. processed the data and drew the figures. H.Z. and Z.Y. assisted in fabricating the E-bandage. H.S., F.L., S.D., Z.W., A.X., Z.D., M.L. and D.Y. assisted in the in vitro experiments. S.W., J.C., T.C., K.Y., Yilin Liu and S.K. assisted in the in vivo experiments. L.L., S.K. and Y. Li assisted in simulation. All authors discussed and agreed with the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Electronics thanks Quansan Yang, Zhou Li and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Note 1, Figs. 1–31, Tables 1–6, Videos 1–3 and References.
Supplementary Video 1
The E-bandage in shaking and swing scenarios remained firmly adhered to the intestinal tissue.
Supplementary Video 2
E-bandage attached to the swelling surface of the intestine remained intact.
Supplementary Video 3
Ultrasound image after E-bandage implantation in mouse intestine.
Supplementary Data 1
Source data for Supplementary Figs. 2, 4, 5, 7–9, 11–17, 20–24, 27, 29 and 31.
Source data
Source Data Figs. 2–6
Statistical source data from Figs. 2–6 and unprocessed western blots from Figs. 2 and 3.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, H., Wang, Y., Li, H. et al. Accelerated intestinal wound healing via dual electrostimulation from a soft and biodegradable electronic bandage. Nat Electron 7, 299–312 (2024). https://doi.org/10.1038/s41928-024-01138-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41928-024-01138-8