Abstract
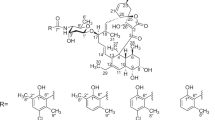
SINCE the introduction in 1961 of 6[D(−)α-amino-phenyl-acetamido]penicillanic acid1 (I; ampicillin), its synthesis, bacteriology, pharmacology, and clinical use as a broad-spectrum penicillin have been extensively reported. Recently, attention has been turned to the physical properties of the solid. Thus, Grant and Alburn2 have distinguished between, a crystalline anhydrous form and a monohydrate, which differ in solid-state infra-red absorption spectra, density, solubility, and thermal stability. They make no mention, however, of the highly crystalline ampicillin trihydrate which may be obtained by crystallization from water3 or by treating acid addition salts in moist organic solvents with amines of high molecular weight4. We wish to report further details of the various solid forms of ampicillin, both anhydrous and hydrated, and the means whereby they may be inter-converted.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Doyle, F. P., Nayler, J. H. C., Smith, H., and Stove, E. R., Nature, 191, 1091 (1961).
Grant, N. H., and Alburn, H. E., Nature, 207, 645 (1965).
Fosker, G. R., Nayler, J. H. C., and Wilcox, J. A., Brit. Pat. Spec., 991, 586 (1965).
Johnson, D. A., and Hardcastle, G. A., U.S. Pat. Spec., 3,157,640 (1964).
Doyle, F. P., Fosker, G. R., Nayler, J. H. C., and Smith, H., J. Chem. Soc., 1440 (1962). Doyle, F. P., Nayler, J. H. C., and Smith, H., U.S. Pat. Spec., 2,985,648 (1961).
Soulal, M. J., and Woodford, M. C. (unpublished observations).
Rapson, H. D. C., Brit. Pat. applied for, No. 24919 (1963).
Stove, E. R., Brit. Pat. applied for, No. 35626 (1963).
Grant, N. H., and Alburn, H. E., J. Amer. Chem. Soc., 86, 3870 (1964).
Dane, E., and Dockner, T., Chem. Ber., 98, 789 (1965).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
AUSTIN, K., MARSHALL, A. & SMITH, H. Crystalline Modifications of Ampicillin. Nature 208, 999–1000 (1965). https://doi.org/10.1038/208999a0
Issue Date:
DOI: https://doi.org/10.1038/208999a0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.