Abstract
Prenatal cocaine exposure has been associated with disruption in attention and short-term memory in exposed children and in animal models. The biochemical change or changes responsible for these cognitive deficits are not known. An intriguing possibility, however, is that cocaine exposure during development disrupts the morphology or function of the frontal cortex, a region thought to contribute to cognitive and executive functions. In this report, we examined the effects of intravenous prenatal cocaine exposure on the expression of the immediate-early gene, c-fos, in the adolescent offspring to determine potential sites of disruption. The expression of Fos protein was similar in unhandled rats prenatally treated with saline or cocaine. Prenatal cocaine exposed rats that were handled, but not footshocked, however, demonstrated a dramatic selective increase in Fos expression in the ventral and medial prefrontal cortex. A footshock-induced increase in Fos expression in the prefrontal cortex was noted in prenatal saline, but not prenatal cocaine rats. Interestingly, no differences were noted in baseline or footshock-induced increased Fos expression in nuclei of the amygdala in prenatal cocaine and prenatal saline rats, indicating some aspect of the central response to stress appear unchanged. The unusual activation of the neurons of the medial and ventral prefrontal cortex may be a consequence of in utero cocaine exposure that contributes to the reported deficit in cognition.
Similar content being viewed by others
Main
The abuse of cocaine during pregnancy has not been associated with major anatomical birth defects; however, a significant numbers of children exposed to cocaine in utero go on to develop select cognitive deficits, such as memory deficits, impulsivity, and difficulties in modulating attention (Delaney-Black et al. 1998; Dow-Edwards et al. 1999). Behavioral studies in animal models of prenatal cocaine exposure have demonstrated similar deficits in memory (Choi et al. 1998a; Cutler et al. 1996; Heyser et al. 1995; Levin and Seidler 1993; Morrow et al. 2000b; Tonkiss et al. 1997; Vorhees et al. 1995) and attention (Garavan et al. 2000; Mactutus 1999; Romano and Harvey 1996). The identification of the sites and the underlying biochemical changes induced by in utero exposure to cocaine, however, remain elusive. Understanding the biochemical substrates of the behavioral deficits induced by prenatal cocaine is necessary if logical treatments for the condition are to be developed. We have chosen to use the expression of the immediate-early gene, c-fos, as a marker of neuronal activation. Many researchers, including in our own laboratory, have successfully used this marker to map and study stress responses (Kovacs 1998). The expression of Fos, however, does not solely indicate that the neuron has been activated but indicates that the neuron is in the process of undergoing longer-term adaptation brought about by activation.
One potential candidate site for the cognitive effects of prenatal cocaine is the frontal cortex. Dysfunction in this region has been associated with disruption in cognitive processes, including memory and attention (Goldman-Rakic 1998). Additionally, deficits in short-term working memory can be observed with altered dopaminergic function in the prefrontal cortex of rats and non-human primates (Goldman-Rakic 1998; Morrow et al. 2000c; Murphy et al. 1996). One obvious mechanism by which prenatal cocaine exposure could impact on the frontal cortex is by blockade by cocaine of the monoamine transporters, including that of dopamine, in that region. Fetal changes in monoamine levels in the developing frontal cortex could potentially result in an anatomical or functional disruption (Levitt 1998; Pendleton et al. 1998). Wang and colleagues reported changes in the GABAergic neurons in the cortex, leading to the hypothesis that prenatal cocaine exposure could result in a disruption of the excitatory/inhibitory balance (Wang et al. 1995, 1996). In this current report, we investigate potential changes in the activation of the intrinsic neurons of the frontal cortex using Fos as a marker.
A portion of the frontal cortex, specifically the medial prefrontal cortex in the rat, has been indicated to be sensitive to stress. Exposure to footshock resulted in increased expression of the immediate early gene, c-fos, in the medial prefrontal cortex compared with other frontal cortical regions (Morrow et al. 2000a). In addition to the intrinsic frontal cortex neurons, the dopaminergic projections to this region are responsive to environmental stress, see (Roth et al. 1988) for review. With regards to prenatal cocaine exposure, Spear and colleagues (Katovic et al. 1999; Wood and Spear 1998), and Soliman and colleagues (Choi et al. 1998b; Huber et al. 2001), have both hypothesized that prenatal cocaine altered an animal's response to stress. To test the extent of this hypothesized disruption in the normal stress response, we have chosen to also examine the amygdala—a specialized cortical region associated with responsiveness to stress and memory involving rewards/punishments (for review see LeDoux 1993, 2000).
EXPERIMENTAL PROCEDURES
Cocaine Administration to Pregnant Dams
Cocaine was administered to individually housed, pregnant rats for 11 days through an intravenous indwelling catheter starting embryonic day E 10 and ending with E 20. On E 7 or 8, timed pregnant Sprague-Dawley dams (Charles River Laboratory, Wilmington, MA) were anesthetized using halothane. Incision sites on the neck and back were shaved and scrubbed with a povidine-iodine solution. A small incision was made on the back and neck and a subcutaneous tunnel for the catheter was made between the incisions. The right external jugular vein was isolated and catheterized with PE-50 tubing that was pushed to the approximate level of the right atrium. The catheter was prepared with attached “dumb-bell” shaped PE-90 tubing that was used to secure the catheter in place with suture (Waynforth and Flecknell 1992). The neck wound was closed with suture. The other end of the catheter was connected to an injection port that was previously imbedded in acrylic resin to allow for proper orientation in the animal's back. The wound on the animal's back was closed with staples leaving the silastic end of the injection port exposed. A topical antibiotic/analgesic (neomycin, polymyxin B, bacitracin, and pramoxine; Schein, Port Washington, NY) was applied to both wounds and the animals were given acetaminophen (1.6 mg/ml; Schein) in the drinking water for 24 h after surgery. The catheter was flushed with heparin/saline (20 units/ml) twice a day until cocaine was administered.
Starting on E 10, two or three days after surgery, surgically prepared rats received saline or cocaine (3 mg/kg) twice a day at approximately 10 A.M. and 4 P.M. Rats were weighed daily to assure accurate dosage and the injection port was cleaned with a 70% ethanol scrub before each injection. Sterile supplies and drugs were used at all times. The injection volume was 1 ml/kg and was delivered over a 30 s interval. After the drug administration, the catheter was flushed with heparin saline (20 units/ml), again taking care to avoid a rapid delivery of the remaining cocaine in the line. Drug administration was stopped after E 20 to: (1) avoid any potential cocaine or handling induced complications with the delivery of the pups on approximately E 21 or 22; (2) assure that each dam received the same dosage of cocaine regardless of when the delivery occurred.
Within 24 h after birth, the pups from the saline and cocaine treated dams were moved to foster dams that had also just given birth. The number of pups was reduced to 11 per group, if necessary, and the pups were left undisturbed until postnatal day P 21, when they were weaned from the foster dams and separated by sex. Rats were group housed with their same-sex littermates with a maximum of five rats in a cage, however no rat was individually housed and only one rat was used per litter for each treatment group. After weaning, the rats were left undisturbed until testing started at P 41–42. After fostering, the surgically prepared dams were given an overdose of pentobarbital (100 mg/kg) and the location of the tip of the catheter was verified during a necropsy examination. All animal procedures were approved by the Yale University Institutional Animal Care and Use Committee and designed to minimize animal use and suffering.
Experimental Design
Two adolescent (P 41 or 42) male offspring from each of four saline litters and four cocaine litters were moved to the laboratory from the animal facility and tested for the effects of footshock on the expression of the immediate-early gene, c-fos (Morrow et al. 2000a). The pre-adult age was selected to better mimic the effects observed in children (Delaney-Black et al. 1998; Dow-Edwards et al. 1999) and to be comparable to other work done in our laboratory (Elsworth et al. 2000; Morrow et al. 2000b). Rats were habituated to a novel chamber (24 × 30 × 27 cm) for 30 min during each of three daily sessions based upon previous experience showing that this protocol would suppress novelty-induced Fos expression in untreated male rats. One rat of each litter was subjected to a mild footshock, ten randomly timed 0.8 mA shocks each paired with a soft tone, over 30 min, as previously published (Morrow et al. 1995, 1999b, 2000a), while the other rat from each litter was subjected to randomly timed tones without the footshock. The tone was produced by a piezoelectric cell, 2.8 kHz and was 5 dB above background noise. This tone did not result in any startle response in the rat, as previously reported (Morrow et al. 1995). Rats exposed to this tone/footshock protocol do not generally jump or display overt escape behaviors, but tend to remain immobile for an increasing interval after the tone/footshock, starting at about 5–10 s after the first tone/footshock and increasing to about 40–50 s after the final tone/footshock (Morrow et al. 1995, 1999b, 2000a). The chamber was cleaned between animals with 70% ethanol and was contained within a dimly lit sound attenuating chamber with a white noise generator to minimize external noises. After a 2 h delay to allow for the production of Fos protein, rats were give pentobarbital (65 mg/kg i.p.; Sigma, St. Louis, MO) to induce deep anesthesia and received trans-cardiac perfusion with 50 ml of saline with heparin (1 unit/ml) followed by 250 ml 4% formaldehyde in phosphate buffer (PB; 0.1 M, pH=7.4).
One male offspring from each of three cocaine and three saline litters was left in the animal facility until approximately 5 min before they were killed on P 41 to provide a measure of Fos-ir expression under the animal's normal conditions. These rats were born and remained in the same room of the housing facility until they were removed from the room, anesthetized with pentobarbital, and perfused as previously described. This procedure prior to fixation took approximately 5 min, which is insufficient time for the production of handling-induced Fos protein.
Immunocytochemical Analysis
The brains were stored overnight in 4% formaldehyde in PB and then cut on a vibratome into 50 μm sections and serially separated into five sets of tissues, so that each set had tissue sections 250 μm (5 × 50 μm) apart. A set of tissue sections was immunostained using immunocytochemistry techniques previously described (Morrow et al. 1999a, 2000a). Briefly, prepared sections were incubated in a rabbit anti-Fos antiserum (1:20,000 dilution, Ab-5, Oncogene Science, Cambridge, MA) overnight at room temperature. Fos-ir was visualized using the ABC technique (Hsu et al. 1981) using a Vectastain Elite kit (Vector Labs, Burlingame, CA) as follows: (1) incubation for 2 h with a biotinylated goat anti-rabbit secondary antibody (Vector Labs, 1:600 dilution in PB); (2) rinsed with PB (3 times for 10 min); (3) incubated in an avidin-biotin complex reagent for 2 h at room temperature. The sections were then washed and the tissue-bound peroxidase was visualized using a nickel-intensified diaminobenzidine reaction (0.04% diaminobenzidine, 2.5% nickel sulfate, & 0.005% hydrogen peroxide. Finally, sections were washed repeatedly with PB to terminate the reaction. An adjacent set of tissue sections was immunostained for Fos and then stained using 0.1% Cresyl Violet, a Nissl stain, to allow visualization of brain landmarks. All sections were mounted on gelatin coated slides and covered with coverslips. Black and white photomicrographs in Figure 1 and 2 demonstrate both the Fos-ir immunostaining and cresyl violet counterstain. Each batch of antibody, primary or secondary, used was tested for non-specific staining by omission of the appropriate antibody.
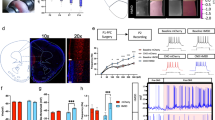
Photocollage of Fos-immunoreactive staining in cortical regions of a rat exposed to cocaine in utero. Panel A: Overview of brain section with the ventral and lateral orbital (VO and LO, respectively), infralimbic (IL), prelimbic (PL), anterior cingulate (aCg), and primary (M1) and secondary (M2) motor cortices identified. Panel B: Low magnification photomicrograph of the PL region only stained for Fos-ir. The white matter (wm) and cortical layers I and VI are identified. Panel C: A high magnification photomicrograph of Fos-ir nuclei (black) in cresyl violet stained cells (gray).
Fos-ir nuclei were counted using a modified single section dissector method based on published methods (Howard and Reed 1998). The density of Fos-ir nuclei in the areas of interest were determined using a microscope (Olympus BH-2), CCD camera/frame grabber board (Scion Corp., Frederick, MD) and Macintosh computer running the public domain software, NIH Image (developed at the U.S. National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image). Cavalieri grids were used with NIH Image from macros developed by Glenn MacDonald at the University of Washington (ftp://codon.nih.gov/pub/nih-image/contrib/Cavalieri_3.sit.bin). The areas of interest were sampled using a systematic random sampling technique. In this portion of the study, only the deep layers (layers V and VI) were sampled as stress has been shown to strongly activate Fos-ir in the deep layers of the medial prefrontal cortex (Morrow et al. 2000a). The regions of the frontal cortex were sampled approximately between 2.2 and 3.2 mm anterior to Bregma to allow comparison with the known biochemical activity of dopamine within the prelimbic and infralimbic regions (Morrow et al. 1999b). Cortical regions sampled within the frontal cortex include the prelimbic (PL), infralimbic (IL), anterior cingulate (aCg), lateral orbital (LO), ventral orbital (VO), anterior primary motor (M1), and anterior secondary motor (aM2). All regions were determined from the Nissl stained sections based upon identification using an atlas (Paxinos and Watson 1997). Other cortical regions were sampled from approximately 1.8 to 3.8 mm posterior to Bregma and include the insular, anterior primary somatosensory (aS1), posterior primary somatosensory (pS1), secondary somatosensory (S2), retrosplenial (RSP), and auditory cortices. The density of Fos-ir nuclei was used to adjust for the different sizes of the regions of interest, thus, allowing comparisons across the different cortical regions.
The volume of the PFC cortical regions, where Fos-ir was noted to be most affected, was measured using a Cavalieri point grid technique from the Nissl stained sections. The widths of the layers of medial PFC were measured from the Nissl stained sections drawn using a camera lucida apparatus attached to an Olympus BH-2 microscope. The widths of the layers within the sections were measured at the dorsal edge of the region of interest.
The expression of Fos-ir in the amygdala was determined by simple profile counting at two levels (approximately −2.5 and −2.8 mm to Bregma) using a camera lucida device connected to an Olympus BH-2 microscope. As no differences were noted between the prenatal cocaine and prenatal saline rats, no further attempt was made to estimate the total number of Fos-ir nuclei or regional volumes in the subregions of the amygdala.
Prenatal exposure to cocaine was associated with a dramatic increase in the number of, and variability within a group of, Fos-ir nuclei in the frontal cortex so that these data violated the assumption of homogeneity of variance required for analysis of variance (ANOVA) (Box test for homogeneity of variance, Winer 1971, pp 37–41). To correct this, the data were transformed using a square root transformation (Winer 1971, pp 397–401). A repeated measures, univariate, ANOVA with type III sums of squares was used to analyze the transformed mean density of Fos-ir nuclei in the cortical brain regions with the between factors of prenatal treatment and acute treatment (footshock). The within factor was the cortical region. Main effects were further investigated using Newman-Keuls range testing. Data from the amygdala were analyzed as previously described without the square root transformation. Comparison of the effects of prenatal cocaine exposure in the home environment were performed using a repeated measures, univariate, ANOVA with the between factor being prenatal treatment and the within factor being cortical region. Specific contrasts were performed by means comparison, assigning equal weights to each cell (Winer 1971), when significant interactions of the main effects were observed. p < .05 was considered significant for all tests and all data is presented as mean ± the standard error of the mean.
RESULTS
A number of parameters were monitored throughout the gestational period and in the resulting offspring. No differences were observed in maternal weights or weight gain, gestational duration, number of offspring, or male:female ratio (data not shown, see Morrow et al., in press). The weights of the male pups at P 41 were not altered by cocaine exposure (saline 199 ± 7 g; cocaine 200 ± 6 g). Additionally, no seizures or unexpected behaviors occurred during or after the administration of cocaine or saline in any pregnant dams.
Fos-ir Expression Was Elevated in Non-shocked Prenatal Cocaine Rats
Before density measures of Fos-ir nuclei could be made, we determined if there were any changes in the areas of the regions of interest. A change in the cortical area due to prenatal cocaine treatment would then require a different method of analysis (Howard and Reed 1998). On Nissl stained sections, the volume of subregions of the prefrontal cortex were determined using Cavalieri method. Prenatal exposure to cocaine had no effect on the cortical volumes of these subregions (Table 1) . The thickness of the cortical layers of the mPFC were measured at five intervals of 250 μm from approximately 2.2 to 3.2 mm anterior to Bregma. No differences in cortical layer thickness were noted in any layer at any level of the mPFC (Table 2)
Adolescent male rats prenatally exposed to either cocaine or saline were exposed to a novel, neutral environment for three sessions to reduce novelty related Fos-ir expression in the mPFC, as is our usual protocol (Morrow et al. 2000a). The following day, rats were subjected to 10 soft tones each lasting 5 s. Half of the rats also received a paired footshock (see following section). Figure 1 and 2 demonstrate immunostaining for Fos-ir nuclei, shown as black in these photomicrographs, with a Nissl counterstain, shown as gray. This allowed for clear identification of the number of Fos-ir nuclei as well as identification of anatomical location. Non-shocked, prenatal saline rats show low levels of Fos-ir expression, as expected from studies with untreated controls (Morrow et al. 1999a, 2000a). In the handled, non-shocked rats, prenatal cocaine exposure was associated with dramatically elevated expression of Fos-ir in the orbital cortex (LO and VO), the medial prefrontal cortex (IL, PL, and aCg), see Figure 2 for representative photomicrographs of the PL region and Figure 3 for quantification. Other regions showed a smaller, but still significant, increase in Fos-ir expression (M2 and aS1). There were no differences in Fos-ir expression between the prenatal saline and prenatal cocaine rats in the M1, insular, pS1, S2, RSP, or auditory cortical regions.
Fos-immunoreactive (Fos-ir) expression in cortical regions of handled prenatal saline and prenatal cocaine rats exposed to footshock. Rats were exposed to three habituation sessions in the test environment to suppress novelty-induced Fos-ir, as we have previously done. The following day, the rats were returned to the test cage and exposed to 10 soft tones with or without footshock. Handled, non-shocked prenatal cocaine rats demonstrated dramatically elevated Fos-ir expression in the following cortical regions (prenatal treatment X regions interaction: F12,120 = 37.8, p < .0001): ventral and lateral orbital (VO and LO, respectively; p < .0001), infralimbic (IL, p < .0001), prelimbic (PL; p < .0001), anterior cingulate (aCg, p < .0001), secondary motor cortex (M2; p = .0006), and anterior primary somatosensory (aS1, p = .006). No difference was noted in any other cortical region examined: primary motor cortex (M1), insular (Ins), posterior primary somatosensory (pS1), secondary somatosensory (S2), retrosplenial (RSP), and auditory cortex (Aud). Footshock increased Fos-ir nuclei in the LO, VO, IL, and PL of prenatal saline rats (p < .05). Footshock failed to further increase Fos-ir in these regions in the prenatal cocaine exposed rat but did show a small increase in the M2 (p = .04). * indicates p < .05 vs. same treatment, non-shocked control. ♦ indicates p < .05 vs. handled, non-shocked saline control.
Footshock Associated Fos-ir Expression in Cortical Regions of Prenatal Cocaine Rats
Previously we have demonstrated that this tone-paired footshock design results in increased Fos-ir expression in several regions of medial prefrontal cortex of untreated rats (Morrow et al. 2000a). In this current study, prenatal saline rats demonstrated footshock associated increases in the number of Fos-ir nuclei in the ventral and medial PFC, namely the VO, LO, IL, and PL (Figure 3). Prenatal cocaine rats did not show this pattern of activation of Fos-ir in the prefrontal cortex. No footshock-induced activation was seen in prenatal cocaine treated rats in the ventral and medial PFC, although a significant footshock associated increase in Fos-ir expression was noted in the M2. It is, however, quite possible that due to the elevated number of Fos-ir in the non-shocked prenatal cocaine rat, no additional increase could be elicited by footshock.
Orbital Cortex Fos-ir Expression Was Diminished in Prenatal Cocaine Rats in Their Home Environment
After observing elevated Fos-ir expression in prenatal cocaine rats under conditions where levels would be expected to be low, we killed rats for analysis directly from the home cages in the vivarium. These unhandled rats were born and spent their entire lives in this same room. The dramatic elevation of Fos-ir in the medial and ventral PFC observed in handled, non-shocked prenatal cocaine rats was not seen in unhandled prenatal cocaine rats, although a small, but significant, decrease in Fos-ir expression in the LO and VO was noted (Figure 4). Interestingly, animals in the vivarium displayed higher levels of Fos in the prefrontal cortex than handled, non-shocked rats. We assume that this may be due to the constant interactions with cagemates and the noise of the surroundings, but further work is necessary to investigate this observation.
Fos-immunoreactive (Fos-ir) expression in prenatal cocaine and prenatal saline rats in their home cage in the vivarium. Unlike reported in the handled, non-shocked rats, prenatal cocaine exposure was not associated with an increase in Fos-ir density as noted in Figure 3. In contrast, unhandled prenatal cocaine rats demonstrated a significant decrease in Fos-ir expression in the ventral and lateral orbital cortex (VO and LO, p = .0002 and 0.0043, respectively), but no difference in the medial and other areas of the prefrontal cortex, namely infralimbic (IL), prelimbic (PL), anterior cingulate (aCg), and primary and secondary motor cortices (M1 and M2). Prenatal treatment X region interaction: F6,24 = 2.9, p = .028. * indicates p < .05 vs. saline control.
Prenatal Treatment Did Not Alter Control or Footshock Activated Fos-ir Expression in the Amygdala
The number of Fos-ir nuclei were determined in several subregions of the amygdala of rats prenatally treated with either saline or cocaine. Unlike the prefrontal cortical regions, there was no effect of prenatal treatment on non-shock or footshock Fos-ir expression (Figure 5). In both the prenatal saline and prenatal cocaine rats, footshock increased Fos-ir expression in several subregions of the amygdala, including the lateral nucleus (La) basolateral nucleus (BLa), and central nucleus (Ce). Other regions examined showed no effect of footshock, namely the endopiriform nucleus (En), intraamygdaloid division of the bed nucleus of the stria terminalis (BST-IA), intercalated nucleus (IC), basomedial nucleus (BMe), and cortical nucleus (Co).
Fos-immunoreactive (Fos-ir) nuclei in the amygdala of non-shocked and footshocked prenatal cocaine and prenatal saline rats. Footshock increased Fos-ir expression in several subregions of the amygdala, including the lateral (La, p = .038), basolateral (BLa, p < .0001), and central (Ce, p < .0001). Other areas did not show changes including the endopiriform nucleus (En), intraamygdaloid division of the bed nucleus of the stria terminalis (BST-IA), intercalated nucleus (IC), basomedial nucleus (BMe), and cortical nucleus (Co). Region X footshock interaction: F7,91=6.54, p < .0001. There was no effect of prenatal treatment on Fos-ir expression in the amygdala (F1,13=0.12, p = .73). * indicates p < .05 vs. same treatment, non-shocked control.
DISCUSSION
Cognitive deficits have been reported with prenatal exposure to cocaine in humans and in animals models. Infants exposed to cocaine in utero have demonstrated deficits in memory (Singer et al. 1999) and attention (Eyler et al. 1998; Heffelfinger et al. 1997; Karmel and Gardner 1996). Older children have demonstrated a lessened ability to maintain attention on a vigilance task (Richardson et al. 1996) and greater distractibility on continuous performance tasks (Dow-Edwards et al. 1999; Leech et al. 1999). In animal models, similar deficits have been noted. Prenatal cocaine exposed rats performed more poorly than controls in tests of spatial memory (Choi et al. 1998a; Cutler et al. 1996; Heyser et al. 1995; Levin and Seidler 1993; Tonkiss et al. 1997; Vorhees et al. 1995) and non-spatial memory (Morrow et al. 2000b). Deficits in attention have also been noted (Garavan et al. 2000; Mactutus 1999; Romano and Harvey 1996). The mechanism for this pattern of cognitive deficits associated with in utero cocaine exposure is unknown.
Cognitive deficits, including those involving working memory and attention, have been linked to the frontal cortex (Goldman-Rakic 1998). Although the hypothesis that the cognitive effects of prenatal cocaine result from morphological or functional changes in the frontal cortex is intriguing, limited data from animal models are available to support it. Wang and colleagues (1995) noted an increase in the number of GABAergic neurons in the anterior cingulate region of the frontal cortex, but not in the visual cortex, of rabbits exposed to cocaine in utero. In spite of the elevated number of GABAergic interneurons, no change was noted in several other morphometric measures of the frontal cortex, namely cortical lamination, total cell number, and soma size. In this current study, we too failed to see any morphological changes in the frontal cortex. These same authors also noted an increase in the number of dendrites on parvalbumin containing GABAergic interneurons, leading them to conclude that prenatal cocaine exposure resulted in a disruption of the excitatory/inhibitory balance of the anterior cingulate region of the frontal cortex (Wang et al. 1996). This hypothesis is supported by the results presented in this current report. We have observed a dramatic elevation in the expression of the immediate early gene, c-fos, in handled, non-shocked rats exposed to cocaine in utero.
Fos is the protein product of c-fos, a member of the immediate-early gene family and, in combination with other protein products from the c-jun transcription family, forms heterodimers which bind to the AP-1 site on DNA and, thus, regulate the transcription of other genes (Hoffman et al. 1992; Morgan and Curran 1991). The activation of Fos expression in the CNS has been associated with stressful stimuli (Duncan et al. 1996; Morrow et al. 2000a; Schreiber et al. 1991; Smith et al. 1992) as well as many other stimuli which seem to share a novelty component (Campeau et al. 1991; Graybiel et al. 1990; Mack and Mack 1992; Sharp et al. 1991). The expression of Fos indicates that the neuron is in the process of undergoing long-term adaptation brought about by activation. For this reason, not every neuron undergoing activation will also express Fos. Although it is not possible to determine the exact cause of the activation of Fos-ir in the non-shocked, prenatal cocaine rats, it seems feasible that neurons in the medial and ventral prefrontal cortex did not fully adapt to the novelty of the test environment. A second possibility is that the soft tones used during testing contributed to the abnormal Fos expression. The failure of the neurons in the ventral and medial PFC to habituate or adapt to less important, external stimuli may contribute to a failure to attend to more salient stimuli. Our working hypothesis is that prenatal cocaine exposure renders the neurons of the medial and ventral PFC less able to adapt to the surrounding environment and this failure of the frontal cortex to adapt to extraneous, external stimuli contributes to the cognitive deficit reported.
The mechanism of the activation of Fos in the medial and ventral prefrontal cortex of the non-shock, prenatal cocaine rats is not known at this time; however, ongoing studies present an interesting theory. Recently, we have reported that dopaminergic activity in the medial prefrontal cortex of prenatal cocaine rats is enhanced in response to footshock (Elsworth et al. 2000). Potentially, changes in the function of the mesoprefrontal dopamine neurons could contribute to cognitive deficits, including short-term, working memory (Goldman-Rakic 1998; Morrow et al. 2000c; Murphy et al. 1996). Changes in dopamine function, however, may occur in concert with other changes to fundamentally disrupt the excitatory balance of the frontal cortex or changes in dopaminergic activity may be in response to an already disrupted excitatory activation in the frontal cortex. One neurotransmitter system that may potentially collaborate with dopamine in the disrupted excitatory/inhibitory balance of the frontal cortex is the intrinsic GABAergic interneurons. GABAergic neurons are thought to control excitation in the frontal cortex through a number of types of synaptic interactions involving the inhibitory GABA controlled chloride channel (Gabbott et al. 1997; Lewis 2000). In support of this theory, dopamine neurons have been shown to innervate the GABAergic interneurons of the rat medial prefrontal cortex (Sesack et al. 1995). More work, however, is necessary to better understand the significance of this potential interaction.
Several authors, including our laboratory, have proposed that prenatal cocaine exposure renders rats more susceptible to environmental stress and that this enhanced response to stress could result in the cognitive deficits that have been reported. A key portion of the central response to stress appears to involve activation of neurons in the amygdala (Campeau et al. 1991; Kovacs 1998; LeDoux 2000). The results presented here do not fully support the hypothesis that prenatal cocaine renders all the proposed neuronal systems hyper-responsive to stress, as the stress activation of Fos expression in the amygdala was not altered by prenatal cocaine exposure. Additionally, there was no elevation of Fos expression in the amygdala under handled, non-shocked conditions, as was observed in the medial and ventral prefrontal cortex. The activation of Fos in the amygdala of both prenatal saline and prenatal cocaine rats occurred in the primary input nuclei, La and BLa, and output nucleus, Ce (LeDoux 2000). Although footshock did not further increase Fos expression in the ventral and medial prefrontal cortex of prenatal cocaine rats, this effect could likely be due to the high level Fos expression under non-shock conditions. Other studies have noted enhanced measures of some biochemical markers that have normally been associated with stress responses, including elevated serum corticosterone levels in prenatal cocaine rats (Choi et al. 1998b; Huber et al. 2001) and increased dopamine turnover in the medial prefrontal cortex (Elsworth et al. 2000). From the data at hand, however, it appears that prenatal exposure to cocaine does not simply intensify the normal stress response of an animal but may alter select aspects of that response.
In conclusion, we report that prenatal cocaine selectively activated the expression of the immediate early gene, c-fos, in intrinsic neurons of the ventral and medial PFC. No change was noted in baseline or footshock activated Fos expression in the amygdala, thus indicating that prenatal cocaine exposure did not result in a general hypersensitivity of the normal stress response. The biochemical changes that occur in the PFC in response to the prenatal exposure to cocaine may play a role in the disruption of cognitive processes previously reported.
References
Campeau S, Hayward MD, Hope BT, Rosen JB, Nestler EJ, Davis M . (1991): Induction of the c-fos proto-oncogene in rat amygdala during unconditioned and conditioned fear. Brain Res 565: 349–352
Choi SJ, Mazzio E, Soliman KF . (1998a): The effects of gestational cocaine exposure on pregnancy outcome, postnatal development, cognition and locomotion in rats. Ann N Y Acad Sci 844: 324–335
Choi SJ, Mazzio EA, Reams RR, Soliman KF . (1998b): Gestational cocaine exposure alters postnatal pituitary-adrenal axis activity and stress endurance in rats. Ann N Y Acad Sci 844: 336–345
Cutler AR, Wilkerson AE, Gingras JL, Levin ED . (1996): Prenatal cocaine and/or nicotine exposure in rats: preliminary findings on long-term cognitive outcome and genital development at birth. Neurotoxicol Teratol 18: 635–643
Delaney-Black V, Covington C, Templin T, Ager J, Martier S, Sokol R . (1998): Prenatal Cocaine Exposure and Child Behavior. Pediatrics 102: 945–950
Dow-Edwards D, Mayes L, Spear L, Hurd Y . (1999): Cocaine and development: Clinical, behavioral, and neurobiological perspectives - A symposium report [Review]. Neurotoxicology & Teratology. 21: 481–490
Duncan GE, Knapp DJ, Breese GR . (1996): Neuroanatomical characterization of Fos induction in rat behavioral models of anxiety. Brain Res 713: 79–91
Elsworth JD, Morrow BA, Roth RH . (2000): Prenatal exposure to cocaine enhances reactivity and reduces the number of A10 dopaminergic neurons. Society for Neuroscience Abstracts 26: 268
Eyler FD, Behnke M, Conlon M, Woods NS, Wobie K . (1998): Birth outcome from a prospective, matched study of prenatal crack/cocaine use: II. Interactive and dose effects on neurobehavioral assessment. Pediatrics 101: 237–241
Gabbott PL, Dickie BG, Vaid RR, Headlam AJ, Bacon SJ . (1997): Local-circuit neurones in the medial prefrontal cortex (areas 25, 32 and 24b) in the rat: morphology and quantitative distribution. J Comp Neurol 377: 465–499
Garavan H, Morgan RE, Mactutus CF, Levitsky DA, Booze RM, Strupp BJ . (2000): Prenatal cocaine exposure impairs selective attention: evidence from serial reversal and extradimensional shift tasks. Behav Neurosci 114: 725–738
Goldman-Rakic PS . (1998): The cortical dopamine system: role in memory and cognition. Advances in Pharmacology 42: 707–711
Graybiel AM, Moratalla R, Robertson HA . (1990): Amphetamine and cocaine induced drug-specific activation of the c-fos gene in striosomematrix and limbic subdivisions of the striatum. Proceedings of the National Academy of Science USA 87: 6912–6916
Heffelfinger A, Craft S, Shyken J . (1997): Visual attention in children with prenatal cocaine exposure. Journal of the International Neuropsychological Society 3: 237–245
Heyser CJ, Spear NE, Spear LP . (1995): Effects of prenatal exposure to cocaine on Morris water maze performance in adult rats. Behav Neurosci 109: 734–743
Howard CV, Reed MG . (1998) Unbiased stereology: three-dimensional measurement in microscopy. Springer, Springer
Hoffman GE, Smith MS, Fitzsimmons MD . (1992): Detecting steroidal effects on immediate early gene expression in the hypothalamus. Neuroprotocols 1: 52–66
Hsu SM, Raine L, Franger H . (1981): The use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (peroxidase) procedures. Journal of Histochemistry and Cytochemistry 29: 577–590
Huber J, Darling S, Park K, Soliman KF . (2001): Altered responsiveness to stress and NMDA following prenatal exposure to cocaine. Physiology & Behavior 72: 181–188
Karmel BZ, Gardner JM . (1996): Prenatal cocaine exposure effects on arousal-modulated attention during the neonatal period. Developmental Psychobiology 29: 463–480
Katovic NM, Gresack JE, Spear LP . (1999): Schedule-induced polydipsia: gender-specific effects and consequences of prenatal cocaine and postnatal handling. Pharmacol Biochem Behav 64: 695–704
Kovacs KJ . (1998): c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem Int 333: 287–297
LeDoux JE . (1993): Emotional memory systems in the brain. Behavioural Brain Res 58: 69–79
LeDoux JE . (2000): Emotion circuits in the brain. Annu Rev Neurosci 23: 155–184
Leech SL, Richardson GA, Goldschmidt L, Day NL . (1999): Prenatal substance exposure: Effects on attention and impulsivity of 6-year-olds. Neurotoxicology & Teratology 21: 109–118
Levin ED, Seidler FJ . (1993): Sex-related spatial learning differences after prenatal cocaine exposure in the young adult rat. Neurotoxicology 14: 23–28
Levitt P . (1998): Prenatal effects of drugs of abuse on brain development. Drug Alcohol Depend 51: 109–125
Lewis DA . (2000): GABAergic local circuit neurons and prefrontal cortical dysfunction in schizophrenia. Brain Res Reviews 31: 270–276
Mack KJ, Mack PA . (1992): Induction of transcription factors in somatosensory cortex after tactile stimulation. Molecular Brain Res 12: 141–147
Mactutus CF . (1999): Prenatal intravenous cocaine adversely affects attentional processing in preweanling rats. Neurotoxicol Teratol 21: 539–550
Morgan JI, Curran T . (1991): Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annual Review in Neuroscience 14: 421–451
Morrow BA, Taylor JR, Roth RH . (1995): Prior exposure to cocaine diminishes behavioral and biochemical responses to aversive conditioning: reversal by glycine/N-methyl-D-aspartate antagonist co-treatment. Neuroscience 69: 233–240
Morrow BA, Elsworth JD, Inglis FM, Roth RH . (1999a): An antisense oligonucleotide reverses the footshock-induced expression of fos in the rat medial prefrontal cortex and the subsequent expression of conditioned fear-induced immobility. Journal of Neuroscience 19: 5666–5673
Morrow BA, Elsworth JD, Rasmusson AM, Roth RH . (1999b): The role of mesoprefrontal dopamine neurons in the acquisition and expression of conditioned fear in the rat. Neuroscience 92: 553–564
Morrow BA, Elsworth JD, Lee EJK, Roth RH . (2000a): Divergent effects of putative anxiolytics on stress-induced Fos expression in the mesoprefrontal system. Synapse 36: 143–154
Morrow BA, Elsworth JD, Roth RH . (2000b): Prenatal cocaine disrupts short-term memory and the adaption of expression of the immediate-early gene, Fos, in the prefrontal cortex of adult, male rats. Society for Neuroscience Abstracts 26: 267
Morrow BA, Roth RH, Elsworth JD . (2000c): TMT, a predator odor, elevates mesoprefrontal dopamine metabolic activity and disrupts short-term working memory in the rat. Brain Res Bulletin 52: 519–523
Morrow BA, Elsworth JD, Roth RH . (In press): Prenatal cocaine exposure disrupts non-spatial, short-term memory in adolescent and adult male rats.
Murphy BL, Arnsten AF, Jentsch JD, Roth RH . (1996): Dopamine and spatial working memory in rats and monkeys: pharmacological reversal of stress-induced impairment. Journal of Neuroscience 16: 7768–7775
Paxinos G, Watson C . (1997) The rat brain. Academic Press Inc., Academic Press Inc.
Pendleton RG, Rasheed A, Roychowdhury R, Hillman R . (1998): A new role for catecholamines: ontogenesis. Trends Pharmacol Sci 19: 248–251
Richardson GA, Conroy ML, Day NL . (1996): Prenatal cocaine exposure: effects on the development of school-age children. Neurotoxicology & Teratology 18: 627–634
Romano AG, Harvey JA . (1996): Prenatal exposure to cocaine disrupts discrimination learning in adult rabbits. Pharmacology Biochemistry & Behavior 53: 617–621
Roth RH, Tam SY, Ida Y, Yang JX, Deutch AY . (1988): Stress and the mesocorticolimbic dopamine systems. Ann N Y Acad Sci 537: 138–147
Schreiber SS, Tocco G, Shors TJ, Thompson RF . (1991): Activation of immediate early genes after acute stress. Neuroreport 2: 17–20
Sesack SR, Snyder CL, Lewis DA . (1995): Axon terminals immunolabeled for dopamine or tyrosine hydroxylase synapse on GABA-immunoreactive dendrites in rat and monkey cortex. J Comp Neurol 363: 264–280
Sharp FR, Sagar SM, Hicks K, Lowenstein D, Hisanaga K . (1991): c-fos mRNA, Fos, and Fos-related antigen induction by hypertonic saline and stress. J Neurosci 11: 2321–2331
Singer LT, Arendt R, Fagan J, Minnes S, Salvator A, Bolek T, Becker M . (1999): Neonatal visual information processing in cocaine-exposed and non-exposed infants. Infant Behavior & Development. 22: 1–15
Smith MA, Banerjee S, Gold PW, Glowa J . (1992): Induction of c-fos mRNA in rat brain by conditioned and unconditioned stressors. Brain Res 578: 135–141
Tonkiss J, Shultz PL, Shumsky JS, Galler JR . (1997): Development of spatial navigation following prenatal cocaine and malnutrition in rats: lack of additive effects. Neurotoxicol Teratol 19: 363–372
Vorhees CV, Reed TM, Acuff-Smith KD, Schilling MA, Cappon GD, Fisher JE, Pu C . (1995): Long-term learning deficits and changes in unlearned behaviors following in utero exposure to multiple daily doses of cocaine during different exposure periods and maternal plasma cocaine concentrations. Neurotoxicol Teratol 17: 253–264
Wang XH, Jenkins AO, Choi L, Murphy EH . (1996): Altered neuronal distribution of parvalbumin in anterior cingulate cortex of rabbits exposed in utero to cocaine. Exp Brain Res 112: 359–371
Wang XH, Levitt P, Grayson DR, Murphy EH . (1995): Intrauterine cocaine exposure of rabbits: persistent elevation of GABA-immunoreactive neurons in anterior cingulate cortex but not visual cortex. Brain Res 689: 32–46
Waynforth HB, Flecknell PA . (1992) Experimental and surgical technique in the rat. Academic Press Inc., New York.
Winer . (1971) Statistical principles in experimental design. McGraw-Hill, New York.
Wood RD, Spear LP . (1998): Prenatal cocaine alters social competition of infant, adolescent, and adult rats. Behav Neurosci 112: 419–431
Acknowledgements
We would like to thank Ms. Dorothy Cameron for her excellent technical assistance. This work supported by the National Institute of Drug Abuse through DA-11288.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morrow, B., Elsworth, J. & Roth, R. Male Rats Exposed to Cocaine in utero Demonstrate Elevated Expression of Fos in the Prefrontal Cortex in Response to Environment. Neuropsychopharmacol 26, 275–285 (2002). https://doi.org/10.1016/S0893-133X(01)00359-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/S0893-133X(01)00359-1
Keywords
This article is cited by
-
Prenatal earthquake stress exposure in different gestational trimesters is associated with methylation changes in the glucocorticoid receptor gene (NR3C1) and long-term working memory in adulthood
Translational Psychiatry (2022)
-
Developmental Consequences of Fetal Exposure to Drugs: What We Know and What We Still Must Learn
Neuropsychopharmacology (2015)
-
The effects of prenatal cocaine, post-weaning housing and sex on conditioned place preference in adolescent rats
Psychopharmacology (2014)
-
Repeated phencyclidine in monkeys results in loss of parvalbumin-containing axo-axonic projections in the prefrontal cortex
Psychopharmacology (2007)
-
Habituation reduces novelty-induced FOS expression in the striatum and cingulate cortex
Experimental Brain Research (2005)