Abstract
Psychomotor stimulants and mu opioid agonists are often used together by polydrug abusers, and it has been suggested that this form of polydrug abuse may result from the ability of stimulants and mu agonists to enhance each other's abuse-related effects. To investigate this possibility, the present study examined stimulant-opioid interactions in rhesus monkeys trained to discriminate cocaine. Specifically, the effects of the mu opioid agonists heroin, alfentanil, fentanyl, and morphine administered alone or in combination with cocaine or d-amphetamine were examined in five monkeys trained to discriminate 0.4 mg/kg cocaine (IM) from saline in a two-lever, food-reinforced drug discrimination procedure. When administered alone, the rapid onset mu agonists heroin (0.032–0.32 mg/kg) and alfentanil (0.01–0.1 mg/kg) substituted completely for cocaine in three of five monkeys but produced primarily saline-appropriate responding in the other two monkeys. The slower onset mu agonists fentanyl (0.0056–0.056 mg/kg) and morphine (0.56–10 mg/kg) substituted for cocaine in only one of five monkeys. When administered as pretreatments to cocaine, morphine and fentanyl increased levels of cocaine-appropriate responding produced by low doses of cocaine in some monkeys. Morphine pretreatment also increased levels of cocaine-appropriate responding produced by low doses of amphetamine in some monkeys. However, in other monkeys, morphine and fentanyl pretreatment did not alter the discriminative stimulus effects of cocaine or amphetamine. These results indicate that there are substantial individual difference in the effects of mu agonists in cocaine-discriminating rhesus monkeys. In some monkeys, mu agonists mimic or enhance the discriminative stimulus of cocaine, whereas in other monkeys, mu agonists neither mimic nor enhance the effects of stimulants.
Similar content being viewed by others
Main
Stimulants such as cocaine and opioid agonists such as heroin are among the most widely abused illicit drugs in the United States, and these drugs are increasingly used together in a drug combination known as “speedball” (Substance Abuse and Mental Health Services Administration 1996a,b). Although the use and abuse of stimulant-opioid combinations could result simply from the coincident availability of both drugs, stimulant-opioid combinations may also produce interacting effects that contribute to enhanced abuse potential. Some drug users have reported that stimulant-opioid combinations produce greater euphoric effects than either drug alone or that use of stimulants and opioids in combination ameliorates the undesirable effects of each drug (Brecher 1972; Kosten et al. 1986, 1987). In addition to these anecdotal reports, controlled laboratory studies in humans have found that stimulant-opioid combinations produce subjective effects that may differ quantitatively or qualitatively from the effects produced by either drug alone (Foltin and Fischman 1992; Walsh et al. 1996; Foltin et al. 1995; Preston et al. 1996).
The relatively high incidence of “speedball” abuse has also stimulated preclinical research designed to investigate possible behavioral interactions between stimulants and opioids. For example, we recently examined the effects of heroin administered alone or in combination with cocaine in rhesus monkeys trained to discriminate cocaine from saline (Mello et al. 1995). When heroin was administered alone, it produced a dose-dependent and complete substitution for cocaine in three of the five monkeys tested. In the other two monkeys, heroin produced primarily saline-appropriate responding up to doses that eliminated responding. Thus, there was considerable individual variability in the effects of heroin in these monkeys. When heroin was administered as a pretreatment to cocaine, doses of heroin that did not produce cocaine-like effects usually did not alter the discriminative stimulus effects of cocaine. Higher pretreatment doses of heroin usually produced levels of cocaine-appropriate responding similar to those produced by heroin alone. Taken together, these results suggested that heroin and cocaine produced similar discriminative stimulus effects in at least some monkeys, but that pretreatments with heroin did not consistently enhance the discriminative stimulus effects of cocaine.
The effects of heroin administered alone or in combination with cocaine were antagonized by the mu opioid receptor-selective antagonist quadazocine, suggesting that these effects of heroin were mediated by mu opioid receptors (Mello et al. 1995). Similarly, other studies have also suggested that the effects of heroin in rhesus monkeys are mediated primarily by mu opioid receptors (Bertalmio et al. 1992). However, heroin differs from most other mu opioid agonists in at least two respects. First, heroin is a highly lipophilic drug that rapidly crosses the blood-brain barrier and that consequently has a rapid rate of onset following systemic administration (Oldendorf et al. 1972; Hartvig et al. 1984). Second, heroin has a relatively low affinity for opioid receptors that does not correlate with its relatively high potency in vivo (Inturrisi et al. 1983; Bertalmio et al. 1992). For example, heroin was reported to be 10-fold less potent than morphine in competing for tritiated [D-Ala2, NMePhe4, Gly5-01]-enkephalin (DAMGO) binding in rhesus monkey brain, but 10-fold more potent than morphine in a drug discrimination assay in monkeys (Bertalmio et al. 1992). Findings such as these have led to the hypothesis that heroin produces its opioid agonist effects indirectly by acting as a prodrug that is deacetylated to form the active metabolites monoacetyl morphine and morphine (Way et al. 1960; Inturrisi et al. 1983, 1984; Bertalmio et al. 1992). As a result of heroin's distinctive attributes, it is possible that the effects of heroin in cocaine discriminating monkeys could differ from the effects of other mu agonists. To investigate this possibility, one goal of the present study was to compare the effects of heroin with the effects of the other opioid agonists morphine, fentanyl and alfentanil. As with heroin, the behavioral effects of morphine, fentanyl, and alfentanil are thought to be mediated primarily by mu opioid receptors in rhesus monkeys (Dykstra et al. 1987; Walker et al. 1993; Emmerson et al. 1994; Gerak et al. 1994). In addition, these mu agonists have different rates of onset (e.g., Bertalmio and Woods 1987) and may provide insight into the role of rate of onset in determining the effects of mu agonists in cocaine-discriminating monkeys. To verify differences in rate of onset of these mu agonists for the purposes of the present study, the time course of each agonist was examined in an assay of thermal antinociception in which mu agonists produce robust and reliable antinociceptive effects (Dykstra et al. 1987).
A second goal of this study was to examine the ability of morphine to enhance the cocaine-like stimulus effects of another psychomotor stimulant, d-amphetamine. Cocaine and amphetamine share discriminative stimulus effects in animals (e.g., Colpaert et al. 1979; de la Garza and Johanson 1983, 1985) and subjective effects in humans (Fischman et al. 1976), and amphetamine may be used instead of cocaine as the stimulant component in speedball abuse (Kramer et al. 1967: Langrod 1970; Brecher 1972). However, the effects of mu opioid agonists on the discriminative stimulus effects of amphetamine in rhesus monkeys are unknown.
METHODS
Subjects
Five male rhesus monkeys (Macaca mulatta) were studied in the drug discrimination experiments, and these were the same monkeys that were used in our previous study of the effects of heroin in cocaine-discriminating monkeys (Mello et al. 1995). Two female and two male rhesus monkeys were studied in the warm water tail-withdrawal experiments. All monkeys had an experimental history involving the evaluation of monoaminergic and/or opioid compounds. Monkeys weighed 4.4–10.9 kg and were maintained on a diet of fresh fruit, vegetables, and Lab Diet Jumbo Monkey biscuits (PMI Feeds, Inc., St. Louis, MO). In addition, monkeys in the cocaine discrimination experiments could receive 1-g banana pellets (P.J. Noyes Co., Lancaster, NH) during daily operant sessions. Water was continuously available, and a 12-h light-dark cycle was in effect (lights on from 7 A.M. to 7 P.M.).
Animal maintenance and research were conducted in accordance with the guidelines provided by the National Institutes of Health (NIH) Committee on Laboratory Animal Resources. The facility was licensed by the U.S. Department of Agriculture, and protocols were approved by the Institutional Animal Care and Use Committee. The health of the monkeys was periodically monitored by consulting veterinarians. Monkeys had visual, auditory, and olfactory contact with other monkeys throughout the study. Monkeys also had access to puzzle feeders, mirrors, and chew toys to provide environmental enrichment. Operant procedures provided an opportunity for environmental manipulation and enrichment in the cocaine discrimination monkeys (Line et al. 1989).
Drug Discrimination Procedures
Apparatus
Drug discrimination procedures were identical to those used in our previous studies (e.g., Mello et al. 1995). Each monkey was housed individually in a well-ventilated, stainless steel chamber (56 × 71 × 69 cm). The home cages of all monkeys were modified to include an operant panel (28 × 28 cm) mounted on the front wall. Three square translucent response keys (6.4 × 6.4 cm) were arranged 2.54 cm apart in a horizontal row 3.2 cm from the top of the operant panel. Each key could be transilluminated by red or green stimulus lights (Superbright LEDs). The operant panel also supported an externally mounted pellet dispenser (Gerbrands, Model G5210, Arlington, MA) that delivered 1 g fruit-flavored food pellets to a food receptacle mounted on the cage beneath the operant response panel. Operation of the operant panels and data collection were accomplished with Apple IIGS computers located in a separate room.
Discrimination Training
Discrimination sessions consisted of multiple cycles and were conducted 5 days per week. Each cycle consisted of a 15-min time-out period followed by a 5-min response period. During the time-out period, all stimulus lights were off, and responding had no scheduled consequences. During the response period, the right and left response keys were transilluminated red or green, and monkeys could receive up to 10 food pellets by responding under a fixed ratio (FR) 30 schedule of food presentation. For two of the five monkeys, the left key was illuminated green and the right key was illuminated red. For the other three monkeys, the colors of the response keys were reversed. The center key was not illuminated at any time, and responding on the center key had no scheduled consequences. If all available food pellets were delivered before the end of the 5-min response period, the stimulus lights transilluminating the response keys were turned off, and responding had no scheduled consequences for the remainder of that response period.
On training days, monkeys were given an IM injection of either saline or 0.40 mg/kg cocaine 5 min after the beginning of each time-out period (i.e., 10 min before the response period). After the administration of saline, responding on only the green key (the saline-appropriate key) produced food, whereas following administration of 0.40 mg/kg cocaine, only responding on the red key (the drug-appropriate key) produced food. Responses on the inappropriate key reset the FR requirement on the appropriate key. Sessions consisted of one to five cycles each day, and if the training dose of cocaine was administered, it was administered only during the last cycle.
During the response period of each cycle, three dependent variables were determined: (1) percent injection-appropriate responding prior to delivery of the first reinforcer [(injection-appropriate responses emitted prior to 1st reinforcer ÷ total responses emitted prior to delivery of 1st reinforcer) × 100]; (2) percent injection-appropriate responding for the entire response period [(injection-appropriate responses emitted during response period ÷ total responses emitted during response period) × 100]; and (3) response rate (total responses emitted during response period ÷ total time stimulus lights were illuminated).
Monkeys were considered to have acquired cocaine discrimination when the following three criteria were met for seven of eight consecutive training session: (1) the percent injection-appropriate responding prior to delivery of the first reinforcer was greater than or equal to 80% for all cycles; (2) the percent injection-appropriate responding for the entire cycle was greater than or equal to 90% for all cycles; (3) response rates during saline training cycles were greater than 1.0 response per second.
Discrimination Testing
Once monkeys met criterion levels of cocaine discrimination, testing began. Test sessions were identical to training sessions except that responding on either key produced food, and test compounds were administered as described below. Three series of experiments were conducted to characterize the effects of mu opioid agonists in these monkeys.
The first series of experiments examined the effects of cocaine, the other central nervous system (CNS) stimulant d-amphetamine, and the mu opioid agonists heroin, alfentanil, fentanyl, and morphine administered alone. Monkeys received an injection 5 min after the beginning of each cycle of a multiple cycle session, and each injection increased the total, cumulative dose of the test drug by 1/4 or 1/2 log units. Each drug was studied in each monkey up to doses that either substituted completely for the cocaine training stimulus (i.e., produced ≥90% cocaine-appropriate responding for the entire cycle) or that decreased responding to ⩽0.1 responses/s. Initially, all substitution studies were conducted using standard cycles consisting of a 15-min time-out period followed by a 5-min response period, and test compounds were administered 10 min before each response period (i.e., a 10-min pretreatment interval). However, parallel studies using a warm-water tail-withdrawal assay of antinociception (see below) indicated that IM–administered fentanyl and morphine had relatively slow rates of onset, suggesting that longer pretreatment intervals may have been required to allow these mu agonists to reach peak effect. Consequently, both fentanyl and morphine were also studied using longer pretreatment intervals designed to assure that response periods began at the time of peak drug effect. Specifically, fentanyl was studied using 16 min pretreatment intervals, and morphine was studied using 32-min pretreatment intervals. Only four monkeys were studied using these longer pretreatment intervals, because one monkey died of unrelated causes before these experiments could be conducted.
Since morphine and fentanyl did not consistently substitute for cocaine, a second series of experiments examined the effects of morphine and fentanyl on the cumulative cocaine dose-effect curve. Both morphine and fentanyl were administered 20 min prior to the administration of the first dose of cocaine (i.e., 30 min prior to the first response period). In addition, the effects of pretreatment with both the opioid antagonist naltrexone and morphine on the cocaine dose-effect curve were determined. In these experiments, naltrexone was administered 15 min before morphine, and morphine was administered 20 min before the first dose of cocaine.
A third series of experiments examined the effects of morphine pretreatment on the cumulative amphetamine dose-effect curve. Morphine doses were administered 20 min before the first dose of amphetamine.
Training sessions were usually conducted on Mondays, Wednesdays, and Thursdays, and test sessions were conducted on Tuesdays and Fridays. Test sessions were conducted only if the three criteria listed above under “criteria for discrimination” were met during the training day immediately preceding the test day. If responding did not meet criterion levels of discrimination performance, then training was continued until criterion levels of performance were obtained for at least 2 consecutive days.
Data Analysis
Individual subject graphs of the percent cocaine-appropriate responding (for the entire response period) and response rates were plotted as a function of the cumulative dose of cocaine (log scale). The percent cocaine-appropriate responding for a given cycle was calculated and reported only if the monkey emitted enough responses to earn at least one food pellet (i.e., 30 responses, equivalent to a response rate of 0.1 responses/s). For experiments examining the effects of cocaine, d-amphetamine and mu opioid agonists administered alone, a drug was considered to have substituted for cocaine in a monkey if at least one dose of the drug elicited ≥90% cocaine-appropriate responding. For experiments examining the effects of mu agonist pretreatments on the cocaine and amphetamine dose-effect curves, the cocaine and amphetamine dose-effect curves following mu opioid pretreatment were visually compared to a range of control dose-effect curves.
Warm-Water Tail-Withdrawal Procedure
Apparatus and Procedure
The results of the initial drug discrimination studies suggested that the ability of mu agonists to mimic the discriminative stimulus effects of cocaine may have been related to their rate of onset (see results below). To provide a direct comparison of the rates of onset of heroin, alfentanil, fentanyl, and morphine following IM administration in rhesus monkeys, the effects of these mu agonists were examined in a warm-water tail-withdrawal assay of antinociception using water heated to 50°C as the noxious stimulus. This assay was used to evaluate time course because mu agonists produce robust and reliable antinociceptive effects in this procedure and because the antinociceptive effects of heroin, alfentanil, fentanyl, and morphine in this procedure are mediated primarily by mu opioid receptors (Dykstra et al. 1987; Walker et al. 1993; Emmerson et al. 1994; Gerak et al. 1994; Negus et al., unpublished observations). The monkeys were seated in acrylic restraint chairs so that their tails moved freely. The bottom 10 cm of each monkey's shaved tail was immersed in a thermal container of warm water. If the subject did not withdraw its tail within 20 s, the tail was removed from the water by the experimenter, and a latency of 20 s was assigned to that measurement. Tail-withdrawal latencies were measured using two different water temperatures: 42 and 50°C. Water heated to 42°C was used as an innocuous control stimulus. Water heated to 50°C was used as the experimental, noxious stimulus. Experiments were conducted no more than twice a week. An Apple IIe microcomputer (Apple Computers, Inc., Cupertino, CA) was used to measure and record time intervals.
Each test session consisted of multiple cycles. Before the first cycle, baseline latencies to tail-withdrawal from 42 and 50°C water were determined. Following preliminary experiments to identify the potency and time course of each agonist, subsequent experiments used two types of testing sequences. During cumulative dosing experiments, a single drug dose was administered at the start of each of four to five sequential cycles, and tail-withdrawal latencies from 42 and 50°C water were measured at the end of each cycle. The two temperatures were presented in a pseudo random order during each cycle. The duration of each cycle was 15 min for alfentanil and heroin and 30 min for fentanyl and morphine. During time course experiments, equi-antinociceptive doses of each mu agonist were administered at the beginning of the session, and tail withdrawal latencies from 50°C water were measured at 2, 4, 8, 16, 32, 64, and 128 min after injection. All drugs were administered IM to mimic the route of administration used in the drug discrimination studies.
Data Analysis
Tail-withdrawal latency values from 50°C water were graphed as a function of both drug dose (for dose-effect studies) and time after drug injection (for time course studies). Equi-effective antinociceptive doses of each drug were identified from the initial dose-effect studies as the lowest dose to produce a mean tail-withdrawal latency of ≥15 s. These equieffective doses were then examined in the time course experiments. Time course data were statistically analyzed using a one-factor ANOVA, with time after injection as the single factor. A significant ANOVA was followed by individual means comparisons using the Newman-Keuls post hoc test (Winer 1971). The criterion for significance was set at p < .01. The time to maximal effect was defined as the interval between the time of drug injection and the earliest time when the following two criteria were met: (1) the mean tail-withdrawal latency was significantly greater than baseline, and (2) tail-withdrawal latencies did not increase significantly at later times.
Drugs
Cocaine hydrochloride, heroin hydrochloride, alfentanil hydrochloride, morphine sulfate, naltrexone hydrochloride (all provided by the National Institute on Drug Abuse, Bethesda, MD), d-amphetamine sulfate (Sigma, St. Louis, MO) and fentanyl citrate (Research Biochemicals International, Natick, MA) were dissolved in sterile water. Doses are expressed as mg/kg of the salt form of the compound. All drugs were administered IM in the thigh.
RESULTS
Warm-Water Tail-Withdrawal Procedure
The relative rates of onset of IM heroin, alfentanil, fentanyl, and morphine were characterized using a warm-water tail-withdrawal assay of thermal antinociception. The monkeys always left their tails in 42°C water for the full 20 s, indicating that tail immersion alone did not elicit tail withdrawal. Baseline tail-withdrawal latencies from 50°C water averaged 1.09 (±0.33) s. Heroin, alfentanil, fentanyl, and morphine all produced dose-dependent increases in tail-withdrawal latencies from 50°C water (Figure 1 , top panel). The lowest doses to elicit tail- withdrawal latencies ≥15 s for each drug were 0.32 mg/kg heroin, 0.1 mg/kg alfentanil, 0.032 mg/kg fentanyl, and 10 mg/kg morphine.
Antinociceptive effects of mu opioid agonists in a warm-water tail-withdrawal assay. Abscissa (top panel): Cumulative dose of drug in mg/kg (log scale). Abscissa (bottom panel): Time in min after injection of drug. Ordinates: Latency in sec to tail withdrawal from water heated to 50°C. Each point shows mean data from three or four monkeys. Points above “BL” in the bottom panel show baseline tail-withdrawal latencies prior to administration of each mu agonist.
The bottom panel of Figure 1 shows the time courses of these equi-effective doses of each mu agonist. Alfentanil and heroin displayed the most rapid onsets of action. Both drugs produced significant (p < .01) and maximal increases in tail-withdrawal latency after 8 min. The effects of alfentanil then decreased rapidly, whereas the maximal effect of heroin was sustained at 16 and 32 min before declining at 64 min. Fentanyl produced a significant but submaximal increase in tail-withdrawal latency after 8 min (p < .01); however, fentanyl did not produce its maximal effect until 16 min. Morphine produced a significant but submaximal increase in tail-withdrawal latency after 16 min, and morphine produced its maximal effect after 32 min. Thus, the order of these drugs was, from fastest to slowest rate of onset, alfentanil = heroin > fentanyl > morphine.
Cocaine Discrimination
Control Performance
During training sessions immediately preceding test sessions, monkeys responded almost exclusively on the saline-appropriate key during saline cycles (95.9 ± 0.4% saline-appropriate responding) and almost exclusively on the cocaine-appropriate key during cocaine training cycles (99.6 ± 0.3% cocaine-appropriate responding). Mean response rates during saline and cocaine training cycles were 2.41 ± 0.16 and 2.04 ± 0.31 responses/s, respectively.
Effects of Mu Agonists Alone
Figure 2 shows the effects of heroin and alfentanil administered alone in individual monkeys. Heroin and alfentanil substituted completely for cocaine in monkeys 89B036, 186F, and 153F. Doses of heroin and alfentanil that produced high levels of cocaine-appropriate responding in these monkeys also usually decreased response rates. Heroin and alfentanil up to doses that eliminated responding produced primarily saline-appropriate responding in monkeys L990 and 150F. Doses of alfentanil (0.032–0.1 mg/kg) and heroin (0.1–0.32 mg/kg) that were behaviorally active in these cocaine discriminating monkeys were identical to doses of these compounds that produced antinociceptive effects in the warm-water tail-withdrawal procedure.
Effects of heroin and alfentanil alone in individual monkeys. Abscissae: Dose drug in mg/kg (log scale). Ordinates (top panels): Percent cocaine-appropriate responding. Ordinates (bottom panels): Response rate in response/s. In this and all other figures, each column of two panels shows data from an individual monkey, and the monkey's identification number is shown above the top panels.
Figure 3 shows the effects of fentanyl and morphine administered alone in the same monkeys. When fentanyl and morphine were administered using the standard 10-min pretreatment interval, neither mu agonist substituted completely for cocaine in any monkey (see open symbols in Figure 3). However, intermediate levels of cocaine-appropriate responding (between 10 and 90%) were occasionally observed, especially in monkey 89B036, and both mu agonists produced dose-dependent decreases in response rates. Since time course studies using the warm-water tail-withdrawal procedure indicated that equieffective IM doses of fentanyl and morphine reached peak effect at 16 and 32 min after administration, respectively, the effects of fentanyl and morphine were subsequently examined using longer pretreatment intervals (16 min for fentanyl and 32 min for morphine; see closed symbols in Figure 3). Under these conditions, both fentanyl and morphine substituted completely for cocaine in monkey 186F, a monkey in which both heroin and alfentanil also substituted for cocaine. In the other monkeys, however, fentanyl and morphine still failed to substitute for cocaine. In fact, fentanyl and morphine produced similar or lower levels of cocaine-appropriate responding in these monkeys when the longer pretreatment interval was used than when the shorter pretreatment interval was used. As with alfentanil and heroin, doses of fentanyl (0.01–0.032 mg/kg) and morphine (3.2–10 mg/kg) that were behaviorally active in the cocaine discriminating monkeys also produced thermal antinociception in the warm-water tail-withdrawal procedure.
Effects of fentanyl and morphine alone in individual monkeys. Abscissae: Dose of drug in mg/kg (log scale). Ordinates (top panels): Percent cocaine-appropriate responding. Ordinates (bottom panels): Response rate in responses/s. Fentanyl and morphine were evaluated using two different pretreatment intervals (i.e., the interval between administration of each dose of drug and the beginning of the subsequent response period during which cocaine-appropriate responding and response rates were evaluated). Fentanyl dose-effect curves were determined using pretreatment intervals of 10 min and 16 min, whereas morphine dose-effect curves were determined using pretreatment intervals of 10 min and 32 min.
Effects of Mu Agonist Pretreatment on Cocaine Discrimination
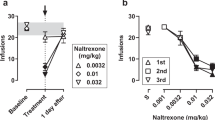
Figure 4 shows the effects of cocaine administered alone or following pretreatment with morphine in each of the five monkeys. Cocaine produced a dose-dependent and complete substitution for the training dose of cocaine in all five monkeys, while having little effect on response rates. Pretreatment with morphine (1.8–3.2 mg/kg) increased levels of cocaine-appropriate responding produced by low doses of cocaine in monkeys 186F and 89B036. Doses of morphine that altered cocaine discrimination in these monkeys also produced substantial decreases in response rates. In monkeys 153F, L990, and 150F, morphine pretreatment (1.8–10 mg/kg) had little or no effect on the cocaine discrimination dose-effect curve, although morphine decreased response rates in these monkeys (for clarity, only the highest two doses of morphine tested are shown for each monkey). The effects of 3.2 mg/kg morphine on cocaine discrimination and response rates in monkeys 89B036 and 186F could be blocked by pretreatment with the opioid antagonist naltrexone (0.1 mg/kg) (Figure 5 ). Naltrexone (0.1 mg/kg) also blocked the rate decreasing effects of 3.2 mg/kg morphine in monkeys 153F, L990, and 150F (data not shown).
Effects of cocaine administered alone or following morphine pretreatment in individual monkeys. Abscissae: Dose of cocaine in mg/kg (log scale). Ordinates (top panels): Percent cocaine-appropriate responding. Ordinates (bottom panels): Response rate in responses/s. The shaded area shows the range of values obtained during four determinations of the dose-effect curve for cocaine alone. Symbols show the effects of cocaine following pretreatment with different doses of morphine. The dotted line in the upper panel for monkey 186F indicates that the monkey did not respond and that a value for percent cocaine responding could not be determined at a dose (0.013 mg/kg) intermediate between the two connected points.
Effects of cocaine administered alone or following pretreatment with morphine or morphine + naltrexone in monkeys 186F and 89B036. Details as in Figure 3.
The effects of pretreatment with fentanyl on the cocaine dose-effect curve were examined in two monkeys, monkeys 186F and 150F (Figure 6 ). These two monkeys were selected for studies with fentanyl, because they represented the different types of responses obtained with morphine pretreatment (i.e., monkey 186F was representative of animals in which morphine enhanced the discriminative stimulus effects of low doses of cocaine, whereas monkey 150F was representative of monkeys in which morphine did not alter the discriminative stimulus effects of cocaine). Like morphine, fentanyl increased levels of cocaine-appropriate responding produced by low doses of cocaine in monkey 186F, but had no effect on cocaine discrimination in monkey 150F. A higher dose of fentanyl (0.1 mg/kg) eliminated responding in monkey 150F (data not shown).
Effects of cocaine administered alone or following fentanyl pretreatment in monkeys 186F and 150F. Details as in Figure 3.
Effects of Morphine on the Cocaine-Like Discriminative Stimulus Effects of Amphetamine
Figure 7 shows the effects of amphetamine administered alone or after pretreatment with morphine. Like cocaine, amphetamine produced a dose-dependent and complete substitution for the training dose of cocaine in all five monkeys. Morphine (1.0–3.2 mg/kg) increased levels of cocaine- appropriate responding produced by low doses of amphetamine in monkeys 89B036, 186F, L990, and 153F. However, morphine pretreatment had little effect on the cocaine-like discriminative stimulus effects of amphetamine in monkey 150F. Morphine produced dose-dependent decreases in response rates relative to amphetamine alone in all monkeys.
Effects of d-amphetamine administered alone or following morphine pretreatment in individual monkeys. Abscissae: Dose amphetamine in mg/kg (log scale). Ordinates (top panels): Percent cocaine-appropriate responding. Ordinates (bottom panels): Response rate in responses/s. The shaded area shows the range of values obtained during two determinations of the dose-effect curve for amphetamine alone. Open symbols show the effects of amphetamine following morphine pretreatment.
DISCUSSION
Cocaine-Like Stimulus Effects of Mu Agonists
In the present study, we replicated our earlier finding that heroin substitutes completely for cocaine in some monkeys (Mello et al. 1995). Importantly, heroin produced complete substitution in the same three monkeys (186F, 89B036, and 153F) as in the original study, whereas heroin failed to substitute for cocaine in the other two monkeys (L990 and 150F). Thus, although there are individual differences in the ability of heroin to produce cocaine-like discriminative stimulus effects, the effects of heroin in any one monkey appear to be consistent through time. The reasons for these individual differences are not clear; however, these individual differences were probably not the result of different behavioral histories, because the behavioral histories of the five monkeys used in the discrimination studies were nearly identical.
We extended these findings by examining the effects of the other mu opioid agonists alfentanil, fentanyl, and morphine. Alfentanil produced a dose-dependent and complete substitution for cocaine in the same three monkeys that discriminated heroin as cocaine (monkeys 186F, 89B036, and 153F). Fentanyl and morphine produced lower levels of cocaine-appropriate responding than heroin and alfentanil, and complete substitution of fentanyl and morphine for cocaine was observed in only one monkey (186F). These studies provide additional evidence to suggest that there are individual differences in the effects of mu agonists in cocaine-discriminating rhesus monkeys.
The differential ability of heroin, alfentanil, fentanyl, and morphine to mimic the discriminative stimulus effects of cocaine may be related to their different rates of onset. We evaluated the rates of onset of these mu agonists in a warm-water tail-withdrawal assay of thermal nociception. As noted above in the Methods section, this assay was used to evaluate time course because mu agonists produce robust and reliable antinociceptive effects in this procedure and because the antinociceptive effects of heroin, alfentanil, fentanyl, and morphine in this procedure are mediated primarily by mu opioid receptors. (Dykstra et al. 1987; Walker et al. 1993; Emmerson et al. 1994; Gerak et al. 1994; Negus et al., unpublished observations). When equi-antinociceptive IM doses of each mu agonist were compared, heroin and alfentanil displayed the most rapid rates of onset, whereas fentanyl and morphine had slower rates of onset. The rates of onset of these mu agonists after IM administration have not been directly compared before; however, these results concur with previous reports of the time courses of these mu agonists in rhesus monkeys (Bertalmio and Woods 1987; Gatch et al. 1995) and humans (Reichle et al. 1962; Kaiko et al. 1981; Jaffe and Martin 1990). The ability of the rapid onset mu agonists heroin and alfentanil to produce higher levels of cocaine- appropriate responding than the slower onset mu agonists fentanyl and morphine suggests that rate of onset may be one determinant of the ability of mu agonists to substitute for the discriminative stimulus effects of cocaine.
Many previous studies have found that mu agonists usually do not substitute for cocaine in subjects trained to discriminate cocaine from saline (Colpaert 1978; Dykstra et al. 1992; Spealman and Bergman 1992, 1994; Broadbent et al. 1995). However, as in the present study, there have been exceptions to this general finding, and these exceptions may also be related to the rate of onset of mu agonist effects. For example, morphine administered IM failed to substitute for cocaine in several studies, including the present one. However, morphine administered via the IV route, which produces immediate absorption and rapid distribution, produced high levels of cocaine-appropriate responding (80–100%) in two monkeys trained to discriminate IV cocaine from saline (Ando and Yanagita 1978). Similarly, morphine failed to substitute for cocaine in rats, but fentanyl, which has a more rapid rate of onset, substituted completely for cocaine in six of 10 rats tested (Colpaert 1978). Taken together, these findings suggest that mu agonists may mimic the discriminative stimulus effects of cocaine in some subjects, especially when the onset of mu agonist effects is rapid.
In our previous study, we found that the cocaine- like stimulus effects and rate-decreasing effects of heroin were antagonized by the mu-selective antagonist quadazocine, suggesting that these effects of heroin were mediated by mu opioid receptors (Mello et al. 1995). In the present study, the mu-selective agonist alfentanil was approximately 3- to 10-fold more potent than heroin in producing cocaine-like stimulus effects. Moreover, all four mu agonists produced dose-dependent decreases in response rates, and the potency order of these drugs in decreasing response rates was identical to their potency order in producing antinociception in the warm-water tail-withdrawal procedure (fentanyl > alfentanil > heroin > morphine). The similar relative potencies of these drugs in producing antinociception, decreases in response rates, and cocaine-like stimulus effects (for heroin and alfentanil) is consistent with the conclusion that all these effects were mediated by a common pharmacological mechanism of action, probably activation of mu opioid receptors.
Effects of Mu Agonists in Combination with Cocaine
Although morphine and fentanyl did not consistently substitute for cocaine when administered alone, both mu agonists increased levels of cocaine-appropriate responding produced by low doses of cocaine in some monkeys. However, as with the discriminative stimulus effects of mu agonists alone, there were striking individual differences in the effects of mu agonists administered as pretreatments to cocaine. At one extreme, morphine and fentanyl were most effective in shifting the cocaine dose-effect curve upward and to the left in a monkey (186F) in which mu agonists administered alone substituted completely for cocaine. The effects of morphine on both cocaine-appropriate responding and response rates were antagonized by a relatively low dose of the opioid antagonist naltrexone in monkey 186F as well as in monkey 89B036, further suggesting that these effects were mediated by mu opioid receptors. At the other extreme, morphine and fentanyl were ineffective in altering the cocaine dose-effect curve in a monkey (150F) in which even rapid onset mu agonists failed to substitute for cocaine. The individual differences in the effects of morphine and fentanyl on cocaine discrimination were probably not the result of different behavioral histories across monkeys, because as noted above, the behavioral histories of all five monkeys were similar.
Previous studies of the effects of mu agonists on cocaine discrimination have also reported highly variable results. In squirrel monkeys, morphine and several other mu agonists produced leftward shifts in the cocaine discrimination dose-effect curve in all monkeys tested (Spealman and Bergman 1992, 1994). On the other hand, fentanyl did not alter the cocaine discrimination dose-effect curve in rats (Broadbent et al. 1995). In another study in rats, buprenorphine increased levels of cocaine-appropriate responding occasioned by a low dose of cocaine, but decreased levels of cocaine-appropriate responding occasioned by higher doses of cocaine (Dykstra et al. 1992). The effects of mu agonists on cocaine discrimination have not been evaluated in humans; however, several studies have examined the effects of mu agonists on cocaine-induced subjective effects, and the results of these studies are also variable. For example, the subjective effects of acute administration of morphine/cocaine combinations were usually similar to the effects of either morphine or cocaine alone, suggesting that morphine did not consistently enhance the subjective effects of cocaine (Foltin and Fischman 1992). However, methadone maintenance has been reported to enhance the subjective effects of cocaine (Foltin et al. 1995; Preston et al. 1996). Taken together, these results suggest that interactions between mu opioid agonists and cocaine are complex, and that mu agonists do not uniformly enhance the effects of cocaine in all subjects under all conditions.
In contrast to the inconsistent effects that have been obtained with mu opioid agonists, drugs acting as direct or indirect dopamine agonists usually produce high levels of cocaine-appropriate responding when administered alone (Kleven et al. 1990; Spealman et al. 1991) and leftward shifts in the cocaine-dose effect curve when administered in combination with cocaine (e.g., Cunningham and Callahan 1991; Spealman 1996). Indeed, these findings have provided important evidence for the hypothesis that the discriminative stimulus effects of cocaine are mediated primarily by the effects of cocaine on dopaminergic systems.
Effects of Mu Agonists in Combination with Amphetamine
Amphetamine produced a dose-dependent and complete substitution for the cocaine training stimulus in all five monkeys tested in this study. This finding agrees with many previous reports that cocaine and amphetamine produce similar discriminative stimulus effects in animals (e.g., Colpaert et al. 1979; de la Garza and Johanson 1983, 1985). In addition, morphine shifted the dose-effect curve for amphetamine-induced cocaine-appropriate responding upward and to the left in some monkeys. Previous studies have not examined the effects of mu agonists on the discriminative stimulus effects of amphetamine in primates. However, clinical evidence indicates that amphetamines have been abused as the psychostimulant component of speedball combinations (Kramer et al. 1967; Langrod 1970; Brecher 1972), which suggests that combinations of mu opioids and amphetamine may produce interacting effects. In addition, the present findings agree with previous reports that mu agonists and amphetamine produced additive or greater than additive effects on antinociception (Ahmed et al. 1970; Richards 1975) and on rates of behavior maintained under some schedules of food reinforcement (Lucot et al. 1979). However, it should be noted that mu agonists and amphetamine do not always enhance each other's effects (e.g., Fog 1970), and in one interesting exception, amphetamine decreased the discriminative stimulus effects of morphine in rats trained to discriminate a low dose (3.2 mg/kg), but not a high dose (5.6 mg/kg), of morphine from saline (Young et al. 1992).
In the present study, morphine appeared to be more effective in enhancing the cocaine-like stimulus effects of amphetamine than in enhancing the effects of cocaine itself. In monkeys 186F and 89B036, morphine increased the stimulus effects of both cocaine and amphetamine, but morphine/amphetamine combinations produced less severe rate-decreasing effects than morphine/cocaine combinations. As a result, the effects of morphine could be observed across a broader range of amphetamine doses. In monkeys L990 and 153F, morphine at doses up to 10 mg/kg had little or no effect on the cocaine dose-effect curve, but morphine at doses of 1–3.2 mg/kg produced clear leftward and/or upward shifts in the amphetamine dose-effect curves. Morphine failed to alter the amphetamine dose-effect curve only in monkey 150F, a monkey in which mu agonists also failed to alter the cocaine dose-effect curve. It is not clear why morphine enhanced the cocaine-like discriminative stimulus effects of amphetamine more than those of cocaine itself. Although cocaine and amphetamine share many common physiological and behavioral effects, they have different mechanisms of action. Specifically, cocaine acts primarily by blocking the reuptake of the monoamine neurotransmitters dopamine, norepinephrine, and serotonin (Koe 1976; Taylor and Ho 1978), whereas amphetamine blocks the reuptake and promotes the release of monoamine neurotransmitters (Coyle and Snyder 1969; Taylor and Ho 1978; Langer and Arbilla 1984). It is possible the morphine interacts to a greater degree with monoamine releasers than with monoamine uptake blockers. Furthermore, previous studies have suggested that endogenous opioid systems may be differentially involved in modulating the effects of cocaine and amphetamine. For example, the opioid antagonist naloxone blocks many neurochemical and behavioral effects of amphetamine in rats, but is less effective in blocking the effects of cocaine (Jones and Holtzman 1994; Schad et al. 1995). Indeed, one study found that naloxone attenuated the locomotor activating effects of amphetamine but enhanced the locomotor activating effects of cocaine. These results were interpreted to suggest that endogenous opioid systems differentially regulate the behavioral effects of amphetamine and cocaine. The findings of the present study suggest that exogenous opioid agonists may also differentially regulate the behavioral effects of these two psychomotor stimulants.
Conclusions
The results of the present study demonstrate that there is considerable individual variability in the effects of mu agonists in rhesus monkeys trained to discriminate cocaine from saline. At one extreme, mu agonists (and especially rapid onset mu agonists) produced high levels of cocaine-appropriate responding, and slower onset mu agonists enhanced the effects of cocaine and amphetamine. At the other extreme, mu agonists neither substituted for cocaine nor altered the effects of cocaine or amphetamine. The observed degree of variability suggests that the behavioral interactions between CNS stimulants and mu opioid agonists in rhesus monkeys are complex. Moreover, one implication of these findings is that, in humans, different individuals may be differentially sensitive to the abuse-related effects of stimulant/opioid “speedball” combinations.
References
Ahmed SS, Abraham GJS, Assari M . (1970): Dose-dependent modification of codeine analgesia by d-amphetamine in albino rats. Arch Int Pharmacodyn Ther 184: 240–244
Ando K, Yanagita T . (1978): The discriminative stimulus properties of intravenously administered cocaine in rhesus monkeys. In Colpaert FC, Rosecrans JS (eds), Stimulus Properties of Drugs: Ten Years of Progress. Amsterdam, Elsevier, pp 125–136
Bertalmio AJ, Medzihradsky F, Winger G, Woods JH . (1992): Differential influence of N-dealkylation on the stimulus properties of some opioid agonists. J Pharmacol Exp Ther 261: 278–284
Bertalmio AJ, Woods JH . (1987): Differentiation between mu and kappa receptor-mediated effects in opioid drug discrimination: Apparent pA2 analysis. J Pharmacol Exp Ther 243: 591–597
Brecher EM . (1972): Licit and Illicit Drugs. Boston, Little, Brown and Co.
Broadbent J, Gaspard TM, Dworkin SI . (1995): Assessment of the discriminative stimulus effects of cocaine in the rat: Lack of interaction with opioids. Pharmacol Biochem Behav 51: 379–385
Colpaert FC . (1978): Discriminative stimulus properties of narcotic analgesic drugs. Pharmacol Biochem Behav 9: 863–887
Colpaert FC, Niemegeers CJE, Janssen PAJ . (1979): Discriminative stimulus properties of cocaine: Neuropharmacological characteristics as derived from stimulus generalization experiments. Pharmacol Biochem Behav 10: 535–546
Coyle JT, Snyder SH . (1969): Catecholamine uptake by synaptosomes in homogenates of rat brain: Stereospecificity in different areas. J Pharmacol Exp Ther 170: 221–231
Cunningham KA, Callahan PM . (1991): Monoamine reuptake inhibitors enhance the discriminative state induced by cocaine in the rat. Psychopharmacology 104: 177–180
de la Garza R, Johanson CE . (1983): The discriminative stimulus properties of cocaine in the rhesus monkey. Pharmacol Biochem Behav 19: 145–148
de la Garza R, Johanson CE . (1985): Discriminative stimulus properties of cocaine in pigeons. Psychopharmacology 85: 23–30
Dykstra LA, Doty P, Johnson AB, Picker MJ . (1992): Discriminative stimulus properties of cocaine, alone and in combination with buprenorphine, morphine and naltrexone. Drug Alcohol Depend 30: 227–234
Dykstra LA, Gmerek DE, Winger G, Woods JH . (1987): Kappa opioids in rhesus monkeys. II. Analysis of antagonistic actions of quadazocine and β-funaltrexamine. J Pharmacol Exp Ther 242: 421–427
Emmerson PJ, Liu M-R, Woods JH, Medzihradsky F . (1994): Binding affinity and selectivity of opioids at mu, delta and kappa receptors in monkey brain membranes. J Pharmacol Exp Ther 271: 1630–1637
Fischman MW, Schuster CR, Resnekov L, Schick JFE, Krasnegor NA, Fennell W, Freedman DX . (1976): Cardiovascular and subjective effects of intravenous cocaine administration in humans. Arch Gen Psychiatry 33: 983–989
Fog R . (1970): Behavioral effects in rats of morphine and amphetamine and of a combination of the two drugs. Psychopharmacologia 16: 305–312
Foltin RW, Fischman MW . (1992): The cardiovascular and subjective effects of intravenous cocaine and morphine combinations in humans. J Pharmacol Exp Ther 261: 623–632
Foltin RW, Christiansen I, Levin FR, Fischman MW . (1995): Effects of single and multiple intravenous cocaine injections in humans maintained on methadone. J Pharmacol Exp Ther 275: 38–47
Gatch MB, Negus SS, Butelman ER, Mello NK . (1995): Antinociceptive effects of cocaine/opioid combinations in rhesus monkeys. J Pharmacol Exp Ther 275: 1346–1354
Gerak LR, Butelman ER, Woods JH, France CP . (1994): Antinociceptive and respiratory effects of nalbuphine in rhesus monkeys. J Pharmacol Exp Ther 271: 993–999
Hartvig P, Bergstrom K, Lindberg B, Lundberg PO, Lundqvist H, Langstrom B, Svard H, Rane A . (1984): Kinetics of 11C-labeled opiates in the brain of rhesus monkeys. J Pharmacol Exp Ther 230: 250–255
Inturrisi CE, Max BM, Foley KM, Schultz M, Shin S-U, Houde RW . (1984): The pharmacokinetics of heroin in patients with chronic pain. N Engl J Med 310: 1213–1217
Inturrisi CE, Schultz M, Shin S, Umans JG, Angel L, Simon EJ . (1983): Evidence from opiate binding studies that heroin acts through its metabolites. Life Sci 33: 773–776
Jaffe JH, Martin WR . (1990): Opioid analgesics and antagonists. In Gilman AG, Rall TW, Nies AS and Taylor P (eds), The Pharmacological Basis of Therapeutics. New York, Pergammon, pp 485–521
Jones DN, Holtzman SG . (1994): Influence of naloxone upon motor activity induced by psychomotor stimulant drugs. Psychopharmacology 114: 215–224
Kaiko RF, Wallenstein SL, Rogers AG, Grabinski PY, Houde RW . (1981): Analgesic and mood effects of heroin and morphine in cancer patients with postoperative pain. N Engl J Med 304: 1501–1505
Kleven MS, Anthony EW, Woolverton WL . (1990): Pharmacological characterization of the discriminative stimulus effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther 254: 312–317
Koe BK . (1976): Molecular geometry of inhibitors of the uptake of catecholamines and serotonin in synaptosomal preparations of rat brain. J. Pharmacol Exp Ther 199: 649–661
Kosten TR, Rounsaville BJ, Gawin FH, Kleber HD . (1986): Cocaine abuse among opioid addicts: Demographic and diagnostic factors in treatment. Am J Drug Alcohol Abuse 12: 1–16
Kosten TR, Rounsaville BJ, Kleber HD . (1987): A 2.5 year follow-up of cocaine use among treated opioid addicts. Arch Gen Psychiatry 44: 281–284
Kramer JC, Fischman VS, Littlefield DC . (1967): Amphetamine abuse: pattern and effects of high doses taken intravenously. J Am Med Assoc 201: 305–309
Langer SZ, Arbilla S . (1984): The amphetamine paradox in dopaminergic neurotransmission. Trends Pharamacol Sci 5: 387–390
Langrod J . (1970): Secondary drug use among heroin users. Int J Addict 5: 611–635
Line SW, Markowitz H, Morgan KN, Strong S . (1989): Evaluation of attempts to enrich the environment of single-caged non-human primates. In Driscoll JW (ed), Animal Care and Use in Behavioral Research: Regulations, Issues, and Applications. Beltsville, MD, National Agricultural Library, pp 103–117
Lucot JB, McMillan DE, Leander D . (1979): The behavioral effects of d-amphetamine alone and in combination with acute and chronic morphine treatments in rats. J Pharmacol Exp Ther 210: 158–165
Mello NK, Negus SS, Lukas SE, Mendelson JH, Sholar JW, Drieze J . (1995): A primate model of polydrug abuse: Cocaine and heroin combinations. J Pharmacol Exp Ther 274: 1325–1337
Oldendorf WH, Hyman S, Braun L, Oldendorf SZ . (1972): Blood-brain barrier: Penetration of morphine, codeine, heroin, and methadone after carotid injection. Science 178: 984–986
Preston KL, Sullivan JT, Strain EC, Bigelow GE . (1996): Enhancement of cocaine's abuse liability in methadone-maintained patients. Psychopharmacology 123: 15–25
Reichle CW, Smith GM, Gravenstein JS, Macris SG, Beecher HK . (1962): Comparative analgesic potency of heroin and morphine in postoperative patients. J Pharmacol Exp Ther 136: 43–46
Richards RK . (1975): A study of the effect of d-amphetamine on the toxicity, analgesic potency and swimming impair-ment caused by potent analgesics in mice. Arch Int Pharmacodyn Ther 216: 225–245
Schad CA, Justice JB, Holtzman SG . (1995): Naloxone reduces the neurochemical and behavioral effects of amphetamine but not those of cocaine. Eur J Pharmacol 275: 9–16
Spealman RD . (1996): Dopamine D3 receptor agonists partially reproduce the discriminative stimulus effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther 278: 1128–1137
Spealman RD, Bergman J . (1992): Modulation of the discriminative stimulus effects of cocaine by mu and kappa opioids. J Pharmacol Exp Ther 261: 607–615
Spealman RD, Bergman J . (1994): Opioid modulation of the discriminative stimulus effects of cocaine: comparison of mu, kappa and delta agonists in squirrel monkeys discriminating low doses of cocaine. Behav Pharmacol 5: 21–31
Spealman RD, Bergman J, Madras BK, Melia KF . (1991): Discriminative stimulus effects of cocaine in squirrel monkeys: Involvement of dopamine receptor subtypes. J Pharmacol Exp Ther 258: 945–953
Substance Abuse and Mental Health Services Administration: Preliminary estimates from the 1995 national household survey on drug abuse, Advance Report Number 18 (1996b). Washington, DC, Department of Health & Human Services Publication # SMA96-3107
Substance Abuse and Mental Health Services Administration: Preliminary estimates from the drug abuse warning network, Advance Report Number 17 (1996a). Washington, DC, Department of Health & Human Services Publication # SMA96-3106
Taylor D, Ho BT . (1978): Comparison of inhibition of monoamine uptake by cocaine, methylphenidate and amphetamine. Res Commun Chem Pathol Pharmacol 21: 67–75
Walker EA, Butelman ER, DeCosta BR, Woods JH . (1993): Opioid thermal antinociception in rhesus monkeys: Receptor mechanisms and temperature dependency. J Pharmacol Exp Ther 267: 280–286
Walsh SL, Sullivan JT, Preston KL, Garner JE, Bigelow GE . (1996): Effects of naltrexone on response to intravenous cocaine, hydromorphone and their combination in humans. J Pharmacol Exp Ther 279: 524–538
Way EL, Kemp JW, Young JM, Grassetti DR . (1960): The pharamcologic effects of heroin in relationship to its rate of biotransformation. J Pharmacol Exp Ther 129: 144–155
Winer BJ . (1971): Statistical Principles in Experimental Design. New York, McGraw-Hill, pp 196–202
Young AM, Masaki MA, Geula C . (1992): Discriminative stimulus effects of morphine: Effects of training dose on agonist and antagonist effects of mu opioids. J Pharmacol Exp Ther 261: 246–257
Acknowledgements
The authors thank Peter Fivel, Nicholas Diaz-Migoyo, and Josephine Avery for technical contributions and Elizabeth Hall for providing veterinary oversight. The authors also thank Dr. Bill Morse for his comments on an earlier version of the article. This work was supported by Grants DA 04059, DA 07252, DA 02159 and K05-00101 from the National Institute on Drug Abuse, National Institutes of Health, Bethesda, Maryland.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Negus, S., Gatch, M. & Mello, N. Effects of Mu Opioid Agonists Alone and in Combination with Cocaine and D-Amphetamine in Rhesus Monkeys Trained to Discriminate Cocaine. Neuropsychopharmacol 18, 325–338 (1998). https://doi.org/10.1016/S0893-133X(97)00163-2
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1016/S0893-133X(97)00163-2
Keywords
This article is cited by
-
Interactions between Δ9-tetrahydrocannabinol and μ opioid receptor agonists in rhesus monkeys: discrimination and antinociception
Psychopharmacology (2008)
-
Morphine and Heroin Differentially Modulate In Vivo Hippocampal LTP in Opiate-Dependent Rat
Neuropsychopharmacology (2007)