Abstract
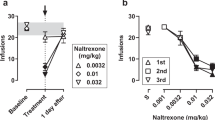
Recent studies suggest that dopamine D3 receptors (D3R) may be a therapeutic target for opioid use disorders (OUD). This study examined the effects of the D3R partial agonist (±)VK4-40 and the D3R-selective antagonist (±)VK4-116, compared to the mu-opioid receptor antagonist naltrexone (NTX), in nonhuman primate models of OUD and antinociception. Adult male and female (N = 4/sex) cynomolgus monkeys were trained to self-administer oxycodone (0.003–0.1 mg/kg/injection) first under a fixed-ratio (FR) and then a progressive-ratio (PR) schedule of reinforcement during daily 1- and 4-hr sessions, respectively. Under the FR schedule, intravenous NTX (0.01–0.1 mg/kg), (±)VK4-116 (1.0–10 mg/kg), and (±)VK4-40 (1.0–10 mg/kg) were studied in combination with the peak oxycodone dose and a dose on the descending limb of the dose-effect curve; NTX and (±)VK4-40 were also studied at the peak of the PR dose-response curve (N = 4). Following saline extinction, each compound was examined on oxycodone-induced reinstatement. Finally, these compounds were assessed in adult male rhesus monkeys (N = 3) in a warm-water (38 °C, 50 °C, 54 °C) tail withdrawal assay. NTX decreased responding on the peak of the FR oxycodone dose-response curve, but increased responding on the descending limb. (±)VK4-40, but not (±)VK4-116, significantly decreased peak oxycodone self-administration; (±)VK4-40 did not increase responding on the descending limb. NTX and (±)VK4-40, but not (±)VK4-116, attenuated oxycodone-induced reinstatement. Under PR responding, NTX and (±)VK4-40 decreased breakpoints. Oxycodone-induced antinociception was attenuated by NTX, but not by (±)VK4-40 or (±)VK4-116. Together, these results suggest that further research evaluating the effects of (±)VK4-40 as a novel pharmacotherapy for OUD is warranted.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kelly RJ, Hearld LR. Service profiles in substance use disorder treatment: are treatment facilities consistent in service mix and service offerings? J Stud Alcohol Drugs. 2022;83:374–83.
Lipari, RN and Van Horn, SL. Trends in substance use disorders among adults aged 18 or older. The CBHSQ Report: June 29, 2017. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, Rockville, MD.
Stephenson J. CDC warns of surge in drug overdose deaths during COVID-19. JAMA Health Forum. 2021;2:e210001–e210001.
Weiner SG, Baker O, Bernson D, Schuur JD. One-year mortality of patients after emergency department treatment for nonfatal opioid overdose. Ann Emerg Med. 2020;75:13–17.
Stringfellow EJ, Lim TY, DiGennaro C, Zhang Z, Paramasivam P, Bearnot B et al. Long-term effects of increasing buprenorphine treatment seeking, duration, and capacity on opioid overdose fatalities: a model-based analysis. J Addict Med. 9900. https://doi.org/10.1097/ADM.0000000000001153.
Shipton EA, Shipton EE, Shipton AJ. A review of the opioid epidemic: what do we do about it? Pain Ther. 2018;7:23–36.
Volkow ND, Collins FS. The role of science in addressing the opioid crisis. N. Engl J Med. 2017;377:391–4.
You Z-B, Bi G-H, Galaj E, Kumar V, Cao J, Gadiano A, et al. Dopamine D3R antagonist VK4-116 attenuates oxycodone self-administration and reinstatement without compromising its antinociceptive effects. Neuropsychopharmacology. 2019;44:1415–24.
Newman AH, Xi Z-X and Heidbreder C. Current perspectives on selective dopamine D3 receptor antagonists/partial agonists as pharmacotherapeutics for opioid and psychostimulant use disorders. Curr Top Behav Neurosci. 2023;60:157–201.
Sokoloff P, Le B. Foll, The dopamine D3 receptor, a quarter century later. Eur J Neurosci. 2017;45:2–19.
Appel NM, Li S-H, Holmes TH, Acri JB. Dopamine D3 receptor antagonist (GSK598809) potentiates the hypertensive effects of cocaine in conscious, freely-moving dogs. J Pharmacol Exp Ther. 2015;354:484–92.
Keck TM, John WS, Czoty PW, Nader MA, Newman AH. Identifying medication targets for psychostimulant addiction: unraveling the dopamine D3 receptor hypothesis. J Med Chem. 2015;58:5361–80.
Kumar V, Bonifazi A, Ellenberger MP, Keck TM, Pommier E, Rais R, et al. Highly selective dopamine D3 receptor (D3R) antagonists and partial agonists based on eticlopride and the D3R crystal structure: new leads for opioid dependence treatment. J Med Chem. 2016;59:7634–50.
de Guglielmo G, Kallupi M, Sedighim S, Newman AH, George O. Dopamine D3 receptor antagonism reverses the escalation of oxycodone self-administration and decreases withdrawal-induced hyperalgesia and irritability-like behavior in oxycodone-dependent heterogeneous stock rats. Front Behav Neurosci. 2020;13:292.
Jordan CJ, Humburg B, Rice M, Bi G-H, You Z-B, Shaik AB, et al. The highly selective dopamine D3R antagonist, R-VK4-40 attenuates oxycodone reward and augments analgesia in rodents. Neuropharmacology. 2019;158:107597.
Jordan CJ, He Y, Bi GH, You ZB, Cao J, Xi ZX, et al. (±) VK4‐40, a novel dopamine D3 receptor partial agonist, attenuates cocaine reward and relapse in rodents. Br J Pharmacol. 2020;177:4796–807.
Doyle MR, Peng LN, Cao J, Rice KC, Newman AH, Collins GT. 3, 4-methylenedioxypyrovalerone high-responder phenotype as a tool to evaluate candidate medications for stimulant use disorder. J Pharmacol Exp Ther. 2023;384:353–62.
Galaj E, Bi G-H, Klein B, Hempel B, Shaik AB, Gogarnoiu ES, et al. A highly D3R-selective and efficacious partial agonist (S)-ABS01-113 compared to its D3R-selective antagonist enantiomer (R)-ABS01-113 as potential treatments for opioid use disorder. Neuropsychopharmacology. 2022;47:2309–18.
Nunes EV, Gordon M, Friedmann PD, Fishman MJ, Lee JD, Chen DT, et al. Relapse to opioid use disorder after inpatient treatment: protective effect of injection naltrexone. J Subst Abus Treat. 2018;85:49–55.
Minhas M, Leri F. A multifaceted analysis of oxycodone addiction. Int J Ment Health Addiction. 2018;16:1016–32.
Becker JB, McClellan ML, Reed BG. Sex differences, gender and addiction. J Neurosci Res. 2017;95:136–47.
Maria MM, Flanagan J, Brady K. Ovarian hormones and drug abuse. Curr psychiatry Rep. 2014;16:1–8.
Back SE, Payne RL, Wahlquist AH, Carter RE, Stroud Z, Haynes L, et al. Comparative profiles of men and women with opioid dependence: results from a national multisite effectiveness trial. Am J drug alcohol Abus. 2011;37:313–23.
Cicero TJ, Aylward SC, Meyer ER. Gender differences in the intravenous self-administration of mu opiate agonists. Pharmacol Biochem Behav. 2003;74:541–9.
Townsend EA, Negus SS, Caine SB, Thomsen M, Banks ML. Sex differences in opioid reinforcement under a fentanyl vs. food choice procedure in rats. Neuropsychopharmacology. 2019;44:2022–9.
Comer SD, Cooper ZD, Kowalczyk WJ, Sullivan MA, Evans SM, Bisaga AM, et al. Evaluation of potential sex differences in the subjective and analgesic effects of morphine in normal, healthy volunteers. Psychopharmacology. 2010;208:45–55.
Cornelissen JC, Obeng S, Rice KC, Zhang Y, Negus SS, Banks ML. Application of receptor theory to the design and use of fixed-proportion mu-opioid agonist and antagonist mixtures in rhesus monkeys. J Pharmacol Exp Ther. 2018;365:37–47.
Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11.
Shaik AB, Kumar V, Bonifazi A, Guerrero AM, Cemaj SL, Gadiano A, et al. Investigation of novel primary and secondary pharmacophores and 3-substitution in the linking chain of a series of highly selective and bitopic dopamine D3 receptor antagonists and partial agonists. J Medicinal Chem. 2019;62:9061–77.
Woolverton WL and Nader MA. Experimental evaluation of the reinforcing effects of drugs. In M. W. Adler, A. Cowan (Eds.), Testing and evaluation of drugs of abuse. John Wiley & Sons; pp. 165–192, 1990.
Panlilio LV, Goldberg SR. Self‐administration of drugs in animals and humans as a model and an investigative tool. Addiction. 2007;102:1863–70.
Levant B. Differential distribution of D3 dopamine receptors in the brains of several mammalian species. Brain Res. 1998;800:269–74.
Nakajima S, Gerretsen P, Takeuchi H, Caravaggio F, Chow T, Le Foll B, et al. The potential role of dopamine D3 receptor neurotransmission in cognition. Eur Neuropsychopharmacol. 2013;23:799–813.
Yager LM, Garcia AF, Wunsch AM, Ferguson SM. The ins and outs of the striatum: role in drug addiction. Neuroscience. 2015;301:529–41.
Sokoloff P, Diaz J, Foll BL, Guillin O, Leriche L, Bezard E, et al. The dopamine D3 receptor: a therapeutic target for the treatment of neuropsychiatric disorders. CNS Neurol Disord-Drug Targets (Former Curr Drug Targets-CNS Neurol Disord). 2006;5:25–43.
Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–69.
Koons AL, Greenberg MR, Cannon RD, Beauchamp GA. Women and the experience of pain and opioid use disorder: a literature-based commentary. Clin Ther. 2018;40:190–6.
Martelle SE, Nader SH, Czoty PW, John WS, Duke AN, Garg PK, et al. Further characterization of quinpirole-elicited yawning as a model of dopamine D3 receptor activation in male and female monkeys. J Pharmacol Exp Ther. 2014;350:205–11.
Moerke MJ, Negus SS, Banks ML. Lack of effect of the nociceptin opioid peptide agonist Ro 64-6198 on pain-depressed behavior and heroin choice in rats. Drug Alcohol Depend. 2022;231:109255.
Acknowledgements
We would like to thank Michael Coller, Jillian H. Odom, Christopher Haggerty and Jianjing Cao for technical assistance.
Funding
This research was supported by DA017763, DA06634, DA037287, T32DA007027 and the NIDA Intramural Research Program Z1ADA000424.
Author information
Authors and Affiliations
Contributions
KW, MLB, AHN and MAN designed the experiment. KW, JCC conducted experiments. MIA conducted data analyses. KW, MIA, MLB, AHN and MAN wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Woodlief, K., Allen, M.I., Cornelissen, J.C. et al. Effects of selective dopamine D3 receptor partial agonist/antagonists on oxycodone self-administration and antinociception in monkeys. Neuropsychopharmacol. 48, 1716–1723 (2023). https://doi.org/10.1038/s41386-023-01590-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-023-01590-8