Abstract
Blockade of dopamine D2 receptors remains a common feature of all antipsychotics. It has been hypothesized that the extrastriatal (cortical, thalamic) dopamine D2 receptors may be more critical to antipsychotic response than the striatal dopamine D2 receptors. This is the first double-blind controlled study to examine the relationship between striatal and extrastriatal D2 occupancy and clinical effects. Fourteen patients with recent onset psychosis were assigned to low or high doses of risperidone (1 mg vs 4 mg/day) or olanzapine (2.5 mg vs 15 mg/day) in order to achieve a broad range of D2 occupancy levels across subjects. Clinical response, side effects, striatal ([11C]-raclopride-positron emission tomography (PET)), and extrastriatal ([11C]-FLB 457-PET) D2 receptors were evaluated after treatment. The measured D2 occupancies ranged from 50 to 92% in striatal and 4 to 95% in the different extrastriatal (frontal, temporal, thalamic) regions. Striatal and extrastriatal occupancies were correlated with dose, drug plasma levels, and with each other. Striatal D2 occupancy predicted response in positive psychotic symptoms (r=0.62, p=0.01), but not for negative symptoms (r=0.2, p=0.5). Extrastriatal D2 occupancy did not predict response in positive or negative symptoms. The two subjects who experienced motor side effects had the highest striatal occupancies in the cohort. Striatal D2 blockade predicted antipsychotic response better than frontal, temporal, and thalamic occupancy. These results, when combined with the preclinical data implicating the mesolimbic striatum in antipsychotic response, suggest that dopamine D2 blockade within specific regions of the striatum may be most critical for ameliorating psychosis in schizophrenia.
Similar content being viewed by others
INTRODUCTION
The definite mechanism of action of antipsychotic medications has not yet been established but antagonism of dopamine transmission plays a central role. Dozens of neuroimaging studies have documented that all antipsychotics (typical and atypical; oral and injectable) block substantial levels of striatal dopamine D2 receptors. The general impression arising from these studies is that a certain level of striatal dopamine D2 blockade, around 60%, is critical for achieving an antipsychotic response and occupancies higher than 80% are associated with motor side effects (Farde et al, 1992; Nordstrom et al, 1993; Kapur et al, 2000). This first generation of positron emission tomography (PET) studies of antipsychotics have focused on measuring striatal (ie caudate and putamen) dopamine D2 occupancy—using tracers such as [11C]-raclopride and [11C]-NMSP with PET and [123I]-IBZM with single-photon emission computerized tomography (SPECT). Dopamine neurons target not only the striatum but also the limbic and cortical areas of the brain and it has been proposed that dopamine action in these extrastriatal (cortical and thalamic) regions, as opposed to the striatum, may be more relevant to antipsychotic action (Bigliani et al, 2000; Xiberas et al, 2001a, 2001b). The focus of this paper is to examine the differential contributions of the dopamine D2 receptors in the striatal vs extrastriatal regions to antipsychotic response.
Measuring extrastriatal D2 occupancy poses a technical challenge, as the levels of dopamine D2 receptors are much lower (a tenth to a hundredth) in extrastriatal regions as compared to the striatum (Kessler et al, 1993). Recently, a number of new tracers with very high affinity for the dopamine D2 receptor have been developed ([11C]-FLB 457 and [18F]-fallypride for PET and [123I]-epidepride for SPECT) making it possible to measure extrastriatal dopamine D2 receptors as well as their occupancy by antipsychotics. Uncontrolled studies using these tracers have led to interesting, but conflicting results. Some studies have reported that atypical antipsychotics, but not typical antipsychotics, show much higher occupancies in extrastriatal relative to striatal regions (Xiberas et al, 2001a, 2001b). Other studies have failed to find such differences (Talvik et al, 2001; Nyberg et al, 2002). Taken together, these studies have demonstrated that it is feasible to measure striatal and extrastriatal receptor occupancy—although none of these studies randomized patients to different occupancies, used standardized prospective treatments, or made prospective longitudinal clinical assessments (Pilowsky, 2001; Xiberas et al, 2001a, 2001b).
We report here the results of the first, systematic, double-blind, prospectively controlled study examining the relationship between striatal and extrastriatal dopamine D2 receptor occupancy and clinical outcome as measured by (a) symptom response, (b) extrapyramidal side effects and akathisia, and (c) prolactin elevation.
METHODS
The study was undertaken at the Centre for Addiction and Mental Health (CAMH), a teaching hospital of the University of Toronto. Eligible subjects were included in the study after they provided written consent using procedures approved by the CAMH Research Ethics Board. The study included patients between the ages of 16 and 55 years who (1) met the DSM-IV criteria for schizophrenia, schizoaffective, or provisional schizophreniform disorder; (2) had never received antipsychotic treatment or had received antipsychotic treatment for less than 4 weeks for the current episode and had been drug free for at least 2 weeks at inclusion; (3) had a positive and negative syndrome scale (PANSS) total score of at least 55 and scored above 3/7 on two or above 4/7 on one of the PANSS-positive subscale item; and (4) had no major medical or neurological illness or history of major head injury. Subjects were excluded if they (1) had a lifetime history of substance dependence or a current diagnosis of substance abuse according to DSM-IV criteria; (2) had a past history of using clozapine or a history of poor response to olanzapine and risperidone.
The diagnoses were established using the MINI (Mini International Neuropsychiatric Interview for DSM-IV and ICD-10) (Sheehan et al, 1998) based on data obtained from the patient supplemented with information from the family and the clinical staff. The patients, in discussion with their treating psychiatrist, chose one of two antipsychotics (risperidone or olanzapine). This option was given to increase the pool of eligible patients, to respect patient choice, and to enhance the generalizability of the results. The patients were then randomized to the low dose (treatment with olanzapine 2.5 mg/day or risperidone 1 mg/day) or standard dose (treatment with olanzapine 15 mg/day or risperidone 4 mg/day) condition of their chosen medication. These doses were chosen on the basis of previous PET occupancy data (Nyberg et al, 1995; Kapur et al, 1997) to give a wide range of dopamine D2 occupancy.
Clinical status was assessed at baseline, on the 7th±1 day and on the 15th±2 days by using the Clinical Global Impression (CGI) Scale (Guy, 1976) for rating improvement and the PANSS (Kay et al, 1987) for assessing clinical symptoms. Akathisia was assessed using the Barnes Akathisia Scale (Barnes, 1989) and parkinsonism was quantified using the Simpson Angus Scale (SAS) (Simpson and Angus, 1970). A rater blind to dose assignment and the PET results assessed clinical status and side effects. Response to treatment was defined as ‘much’ or ‘very much’ improved on the CGI-B improvement scale (Guy, 1976).
Extrastriatal ([11C]-FLB457) and striatal ([11C]-raclopride) dopamine D2 receptor occupancy were assessed after 15th±2 days of continuous antipsychotic treatment 12 and 14 h after the last administered dose of antipsychotic, respectively. PET scanning was conducted using a brain-only PET scanner (GEMS PC2048-Plus), which provides 15, 6.5 mm-thick slices with an in-plane resolution of about 5–6 mm in air. The [11C]-FLB457 PET scans for extrastriatal D2 receptor occupancy were obtained following injection of 9.75 (±0.65) mCi of high specific-activity [11C]-FLB457 via single bolus intravenous injection, with emission scans collected over the next 90 min. The [11C]-raclopride PET scans for striatal D2 receptor occupancy were obtained immediately following injection of 9.54 (±1.46) mCi of high specific-activity [11C]-raclopride using a bolus plus infusion protocol intravenously (Houle et al, 1996) with 59% injected as a bolus over 1 min and the rest injected via intravenous infusion over 74 min.
To permit accurate delineation of the brain regions for data analysis, each patient had a magnetic resonance imaging (MRI) scan on a GE-Sigma 1.5 T scanner using a fast spin-echo T1-weighted sequence with 3 mm slice thickness. The MRI scan of each patient was co-registered to their PET scan using RView8 software (Studholme et al, 1997) and regions of interest (ROIs) were drawn using Alice 3.0 (Perceptive Systems, Boulder, Colorado), which allows the rater to draw ROIs on the co-registered MRI image and transfer them onto the PET images. For one subject, who did not undergo an MRI scan, ROIs were directly delineated on the summed PET image. The ROIs were drawn by a single rater (blind to treatment allocation) using previously described boundaries (Bremner et al, 1998) on two axial slices for the cerebellum (which served as a reference region), two axial slices for the striatum (including the caudate and the putamen), two axial slices for the frontal and temporal cortices, and one axial slice for the thalamus. The ROIs were then transferred onto the dynamic PET images using the same software and a regional time–activity curve (TAC) generated for further analysis.
Striatal D2 receptor binding potential (BP) was calculated using two methods, the simplified reference tissue model (SRTM) (Lammertsma and Hume, 1996) and the ratio method (striatum/cerebellum ratio minus one obtained between 30 and 75 min of scanning) and taken as a measure proportional to D2 BP. This timing was chosen based on previous studies showing a very high correlation between the BP derived with this method and analytically derived estimates of D2 BP (r>0.95) (Kapur et al, 1996). The method shows a scan-rescan SD of 6% and has been standardized in our lab with a high intra- and inter-rater reliability (intra-class correlation coefficients: r>0.95) (Kapur et al, 1998). Extrastriatal D2 receptor BP was calculated from the TACs generated from the [11C]-FLB457 scans using the SRTM, using the cerebellum as the reference region and the frontal cortex, temporal cortex, and thalamus as the ROIs (Lammertsma and Hume, 1996; Olsson et al, 1999).
Receptor occupancy was then calculated as the percentage reduction of D2 BP with drug treatment as compared to the expected baseline (100 × {1−[BPdrug scan/BPExpBaseline]}). Age-corrected measures of expected baseline BP were obtained from a previously collected data set of 17 [11C]-raclopride scans and 13 [11C]-FLB 457 scans of antipsychotic-free, sex- and age-matched healthy subjects. The same rater using the same methodology to ensure within-study consistency obtained the data for normal controls. Venous blood was collected for plasma levels of risperidone, 9-hydroxy-risperidone, olanzapine, and prolactin at the time of the PET scans and were analyzed in standard commercial laboratories.
Group data are expressed as mean±SD. Group differences were statistically compared using a Student's t-test, paired and unpaired as appropriate. Correlations between D2 receptor occupancies and clinical outcomes were examined using the Pearson product moment correlation. To examine the sensitivity of the main conclusions to individual data, each patient was dropped from the analysis, one at a time, and the impact of that on the overall results was examined.
RESULTS
Eighteen patients entered the study and 14 completed the protocol. Three patients discontinued the study because of clinical worsening; all had been randomized to the low dose of olanzapine. One patient who was receiving low-dose risperidone changed her mind about participating after 4 days of the study. As no PET data were available for these patients, they were not included in the analysis. Of the 14 who completed the study, 13 were men, 10 were neuroleptic naïve, and four had 1–2 short trials of antipsychotic medications during the year before the study. The patients had a mean age of 27 years (range: 17–45 years) and a mean duration of psychotic symptoms of 98 weeks (range: 4–530 weeks). Eleven of these patients met the criteria for schizophrenia and three met the criteria for provisional schizophreniform disorder at the time of inclusion in the study.
The patients’ symptoms were of marked severity at the beginning of the study as reflected by their scores on the CGI Severity scale (mean score=4.93, SD=0.61), and the PANSS (PANSS total mean=79.9, SD=11.4; PANSS-positive mean=21.1, SD=4; PANSS-negative mean=17, SD=5.3). After 15±2 days of treatment, the mean percent improvement in the PANSS total score was 17.5%±9.3 and the mean percent improvement of the PANSS-positive score was 31.4%±11.5. Five of the patients were treated with risperidone (two at 1 mg/day and three at 4 mg/day) and nine were treated with olanzapine (three at 2.5 mg/day and six at 15 mg/day). At baseline, symptom severity did not differ significantly for those on low vs standard dose or between those treated with risperidone and olanzapine. Two patients on risperidone were receiving benztropine for extrapyramidal side effects at the time of the follow-up PET scan.
The measured D2 occupancies ranged from 50 to 92% in striatal and 4 to 95% in the different extrastriatal regions. Occupancy in striatal and extrastriatal brain regions were significantly higher in the standard dose group than in the low-dose group. Striatal occupancy was significantly correlated with frontal occupancy (r=0.72, p=0.005), temporal occupancy (r=0.82, p<0.0001), and thalamic occupancy (r=0.81, p<0.0001). Striatal occupancy (mean=71.9, SD=16.7) was higher than temporal occupancy (mean=57.7, SD=21, p<0.001) and thalamic occupancy (mean=61.5, SD=19, p=0.005), but did not reliably differentiate from frontal occupancy (mean=65.7, SD=30.5, p=0.2). The extrastriatal occupancies at the frontal, temporal, and thalamic brain regions were not different from each other (F=−1.019; df=2; p=0.376).
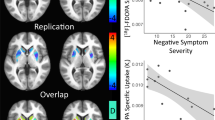
Striatal and extrastriatal occupancy were not correlated with severity of illness at baseline—assuring that the process of randomization yielded appropriate outcomes on this variable. Percent change in PANSS-positive symptoms was significantly correlated with striatal occupancy (r=0.62, p=0.01) (Figure 1) but not with extrastriatal occupancy: r=0.34, p=0.25 (frontal); r=0.30, p=0.29 (temporal); r=0.36, p=0.20 (thalamus) (Figure 2). Percent change in PANSS-negative symptoms was not correlated with striatal occupancy (r=0.2, p=0.5) or extrastriatal occupancy (frontal: r=0.05, p=0.88; temporal: r=0.26, p=0.37; thalamus: r=0.30, p=0.29) (Figure 3).
Two patients received benztropine because of extrapyramidal side effects. Both of them received the standard dose of risperidone (4 mg) and the total score of their SAS was 13 and 8 (mean Simpson Angus total score for the whole study subjects: 3.2 (SD=3.7)). Mean striatal occupancy for these two subjects was 91.5 (SD=0.05), which was significantly higher in comparison with the group of subjects who did not have extrapyramidal side effects (mean=68.6, SD=15.7) (t=5.03, df=11, p<0.001). Analysis of the data without these two individuals showed that the percent change in PANSS-positive symptoms was significantly correlated with striatal occupancy (r=0.6, p=0.04) but not with extrastriatal occupancy: r=0.26, p=0.43 (frontal); r=0.34, p=0.28 (temporal); r=0.31, p=0.32 (thalamus). Percent change in PANSS-negative symptoms was not correlated with striatal occupancy (r=0.22, p=0.5) or extrastriatal occupancy (frontal: r=0.01, p=0.97; temporal: r=0.18, p=0.58; thalamus: r=0.33, p=0.30).
Prolactin levels showed a trend towards correlation with striatal occupancy (r=0.47, p=0.09), but there was no observable relationship to extrastriatal D2 occupancy: r=0.4, p=0.17 (frontal); r=0.26, p=0.36 (temporal); r=0.38; p=0.17 (thalamus).
Given the small sample size, we examined the sensitivity of these findings to individual data. Each subject was dropped from the analysis, one at a time, and the relationship between striatal occupancy and PANSS-P continued to remain significant (p<0.05 in all cases), and in none of the cases did the extrastriatal correlations become positive (p>0.05 in all cases; range of p-values: 0.25–0.88). Further, to examine if the results were an artifact of one method of BP determination, we examined the raclopride data using the specific/nonspecific ratio method, and the main finding of the relationship between striatal occupancy and PANSS-P remained significant (p=0.01) and the other relationships were not significant (p>0.05 in all cases).
DISCUSSION
This is the first double-blind controlled study examining striatal vs extrastriatal D2 occupancy and their relationship to treatment response. As expected from previous data (Nordstrom et al, 1993; Kapur et al, 2000), striatal occupancy predicted improvement in positive symptoms with antipsychotic treatment and was also associated with motor side effects. D2 occupancy in frontal, temporal, and thalamic regions, however, was not associated with any measures of treatment response or side effects.
Before we discuss the possible implications of these findings, we would like to highlight several limitations of this study. First, the sample is small. Having acknowledged that, it should be pointed out that the sample size was determined on the basis of power estimates derived from the previous studies by Nordstrom et al (1993) and Kapur et al (2000) and the results show that the study had sufficient power to replicate the association of striatal D2 occupancy and treatment response. Moreover, despite the small sample size, the results did not change while using the jackknife statistical re-sampling procedure (Efron, 1982). The relationship (correlation or lack of correlation) between response and occupancy remained the same even after excluding the most extreme observations (see Figure 3). Further, analyzing the data using two different methods for BP determination (SRTM and specific/nonspecific ratio method) led to identical results. A second consideration was the relatively short duration of the study. The inclusion of a low-dose group, and the range of occupancies it provided, greatly increased the likelihood of demonstrating an association between D2 occupancy and response. However, given that the low doses used may be less than the optimal for some of the patients, we wanted to keep the period as short as feasible. Thus, a 2-week follow-up period was chosen based on recently published data regarding onset of action of antipsychotics (Agid et al, 2003; Leucht et al, 2005), which demonstrated that the improvement in the first 2 weeks of antipsychotic treatment was significantly higher than in any subsequent 2 weeks of treatment (Agid et al, 2003). Although we have found a linear relationship between D2 occupancy and response, this cannot rule out the existence of a threshold effect. However, considering the small sample size in our study, we can neither confirm nor eliminate this possibility.
Limitations also exist in our estimates of D2 receptor occupancies. To estimate BP for [11C]-FLB457, we used the SRTM—a model that assumes that the cerebellum has no specific binding (Lammertsma and Hume, 1996). Although the estimates of BP derived from SRTM have been shown to be highly correlated with estimates derived from arterial-input based kinetic-modeling, this model may underestimate occupancy because the humans cerebellum shows some displaceable [11C]-FLB457 signal (Hall et al, 1996; Olsson et al, 2004). It is estimated that the error induced by this displaceable [11C]-FLB457 signal in the cerebellum would range from 2 to 5% for true occupancies in the range of 70–90% (Olsson et al, 2004). Although this may have impacted the absolute % occupancy observed here, it is unlikely to have changed the relative order of occupancies observed across. We also noted that [11C]-raclopride occupancies were higher than expected—in our hands similar doses in the past have led to occupancies 5–10% lower (Kapur et al, 1999). One potential explanation might be differences in the plasma levels of the medications during the first ([11C]-FLB 457) and the second ([11C]-raclopride) PET scans. However, there was no significant difference between the plasma levels of the medications between the two consecutive scans (t=1.7; p=0.11; df=13). This is likely due to the fact that the [11C]-raclopride PET scan was performed immediately following the [11C]-FLB 457 PET scan. The very high D2 affinity of [11C]-FLB 457 could lead to a residual ‘cold’ mass effect, thereby artificially exaggerating the occupancy subsequently measured with [11C]-raclopride. Again, although this may increase the absolute % of raclopride measured occupancy, there is no reason to believe this would change the internal ordering across subjects. Finally, we did not have baseline measures of D2 BP for the patients. This was carried out to limit the total number of scans for the patients, especially during the period when they were actively psychotic. As a result, we used an adequate age- and sex-matched sample to provide standard baseline. Lack of the patients’ own baseline values is a potential source of error. For striatal D2 receptor occupancy, this error, as calculated on the basis of variance in the data from antipsychotic-naïve patients, is expected to vary from 0 to 9% for patients with 50% occupancy and 0 to 4% for patients who have 80% occupancy (Farde et al, 1992). However, there is no reason to believe that this would introduce a systematic bias—across ligands, or across drug-doses, which were the main manipulations of interest in this study (Farde et al, 1997; Bigliani et al, 2000; Pilowsky, 2001; Xiberas et al, 2001a, 2001b).
We found that levels of D2 receptor occupancy in the striatum, frontal cortex, temporal cortex, and thalamus were significantly correlated. This is consistent with a number of previous [11C]-FLB457/[11C]-raclopride studies (Talvik et al, 2001; Nyberg et al, 2002) and a recent [18F]-fallypride PET study (Kessler et al, 2003) which have shown similar and correlated levels of striatal and extrastriatal dopamine D2/3 receptor occupancy. However, there are reports by Bigliani et al (2000) and Xiberas et al (2001a, 2001b) that atypical antipsychotic medications demonstrated higher occupancy of extrastriatal cortical brain regions than striatal regions. The reason for this discrepancy between groups reporting similar striatal-extrastriatal occupancy (this study, Talvik et al, 2001; Nyberg et al, 2002; Kessler et al, 2003) and those showing much higher extrastriatal occupancy (Bigliani et al, 2000; Xiberas et al, 2001a, 2001b) are not entirely clear and have been discussed at length elsewhere (Bigliani et al, 2000; Talvik et al, 2001; Xiberas et al, 2001a, 2001b; Nyberg et al, 2002). Issues such as ligand selection, ligand specificity, PET vs SPECT, duration of scanning, non-equilibrium modeling approaches, etc have been raised, but remain unresolved. Regardless of this controversy, none of the previous studies have controlled for, randomly assigned to, or examined the relationship of striatal/extrastriatal occupancy to response. Ours is the first study to do so. And despite a wide range of occupancies as well as a wide range of response outcomes—we fail to find a relationship between extrastriatal occupancy and response. There are two possible explanations: One, a stronger relationship between extrastriatal occupancy does exist but we failed to identify it owing to the limitations of our study (acknowledged above), or alternatively, it could be that D2 occupancy in the striatum is the mediator, not only of EPS, but also of anti-‘psychotic’ response.
It may seem surprising that response of psychosis, a complex emotional and cognitive disturbance, may correlate most strongly to the actions of the drug in the striatum, a region often thought of as just ‘motor’. However, striatal regions impact more than just motor function and their projections to non-motor areas of prefrontal and limbic cortex and are optimally positioned to influence cognitive and emotional networks (Alexander et al, 1986; de Haan, van Bruggen et al, 2003). An example of this was found in a recent study (de Haan, van Bruggen et al, 2003) in which D2 receptor occupancy was discovered to be correlated with the subjective experience of patients with recent onset schizophrenia.
Striatal regions (caudate and putamen) are now accorded a role in the allocation of attention, selection of competing behaviors, and in mediating the influence of motivation and emotion on action (Mogenson et al, 1980; Joel and Weiner, 2000; Haber, 2003). Keeping with this in rodent studies, it is well documented that antipsychotic efficacy of drugs is best predicted by the ability of a drug to alter and/or induce regional gene-induction, selective neuronal depolarization, and regionally selective changes in dopamine turnover in the nucleus accumbens—a ventral region of the striatal complex—as opposed to changes in the dorsal striatum, cortical, or thalamic regions (Arnt et al, 1997; Arnt and Skarsfeldt, 1998). Unlike the rodent, the humans do not have a clearly demarcated nucleus accumbens, but it is thought that the region of the striatum which is anterior and inferior to the anterior commissure is the closest analog of the nucleus accumbens (Chronister and DeFrance, 1981). As in our PET studies this region is captured within the composite signal obtained from the caudate-putamen, it is not surprising that antipsychotic response would correlated most closely with this region.
The resolution of the PET scanner utilized in this study did not allow us to validly differentiate between dorsal and ventral striatum. However, post-mortem studies in humans show that the dorsal and ventral parts of the striatum differ from each other not only by the different afferent inputs they receive and areas they project to (in general, dorsal regions are linked to motor networks, the ventral regions to limibic networks), but also by their neurotransmitter and peptide content, their receptor distribution, their neuronal organization, connectivity, developmental schedule, and behavioral function (Joel and Weiner, 2000; Roberts and Knickman, 2002; Voorn et al, 2004). The data from this study, when combined with neuroanatomical studies as well preclinical data all seem to lead to the hypothesis that dopamine D2 blockade in the ventral striatum, rather than some extrastriatal region, is the critical region for antipsychotic response. The recent advances in high-affinity radioligands and the increased spatial resolution of PET scanners make this an imminently testable hypothesis.
References
Agid O, Kapur S, Arenovich T, Zipursky RB (2003). Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry 60: 1228–1235.
Alexander GE, DeLong MR, Strick PL (1986). Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9: 357–381.
Arnt J, Skarsfeldt T (1998). Do novel antipsychotics have similar pharmacological characteristics? A review of the evidence. Neuropsychopharmacology 18: 63–101.
Arnt J, Skarsfeldt T, Hyttel J (1997). Differentiation of classical and novel antipsychotics using animal models. Int Clin Psychopharmacol 12 (Suppl 1): S9–S17.
Barnes TR (1989). A rating scale for drug-induced akathisia. Br J Psychiatry 154: 672–676.
Bigliani V, Mulligan RS, Acton PD, Ohlsen RI, Pike VW, Ell PJ et al (2000). Striatal and temporal cortical D2/D3 receptor occupancy by olanzapine and sertindole in vivo: a [123I]epidepride single photon emission tomography (SPET) study. Psychopharmacology (Berlin) 150: 132–140.
Bremner JD, Bronen RA, De Erasquin G, Vermetten E, Staib LH, Ng CK et al (1998). Development and reliability of a method for using magnetic resonance imaging for the definition of regions of interest for positron emission tomography. Clin Positron Imaging 1: 145–159.
Chronister RB, DeFrance JF (1981). Nucleus accumbens in historical perspective. In: Chronister RB, DeFrance JF (eds). The Neurobiology of the Nucleus Accumbens. Haer Institute for Electrophysiological Research: Brunswick, Maine. pp 1–6.
de Haan L, van Bruggen M, Lavalaye J, Booij J, Dingemans PM, Linszen D (2003). Subjective experience and D2 receptor occupancy in patients with recent-onset schizophrenia treated with low-dose olanzapine or haloperidol: a randomized, double-blind study. Am J Psychiatry 160: 303–309.
Efron B (1982). The Jackknife, the Bootstrap and Other Resampling Plans. SIAM: Bristol.
Farde L, Nordstrom AL, Wiesel FA, Pauli S, Halldin C, Sedvall G (1992). Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry 49: 538–544.
Farde L, Suhara T, Nyberg S, Karlsson P, Nakashima Y, Hietala J et al (1997). A PET-study of [11C]FLB 457 binding to extrastriatal D2-dopamine receptors in healthy subjects and antipsychotic drug-treated patients. Psychopharmacology (Berlin) 133: 396–404.
Guy W (1976). Clinical Global Impression. US Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, NIMH Psychopharmacology Research Branch, Division of Extramural Research Programs: Rockville, MD.
Haber SN (2003). The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat 26: 317–330.
Hall H, Farde L, Halldin C, Hurd YL, Pauli S, Sedvall G (1996). Autoradiographic localization of extrastriatal D2-dopamine receptors in the human brain using [125I]epidepride. Synapse 23: 115–123.
Houle S, Kapur S, Hussey D, Jones C, DaSilva J, Wilson A (1996). Measurment of [11C]-raclopride binding using a bolus plus infusion protocol. In: Myers R, Cunningham V, Bailey DL, Jones T (eds). Quantification of Brain Function Using PET. Academic Press: San-Diego.
Joel D, Weiner I (2000). The connections of the dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience 96: 451–474.
Kapur S, Remington G, Jones C, Wilson A, DaSilva J, Houle S et al (1996). High levels of dopamine D2 receptor occupancy with low-dose haloperidol treatment: a PET study. Am J Psychiatry 153: 948–950.
Kapur S, Zipursky R, Jones C, Remington G, Houle S (2000). Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry 157: 514–520.
Kapur S, Zipursky RB, Remington G (1999). Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry 156: 286–293.
Kapur S, Zipursky R, Roy P, Jones C, Remington G, Reed K et al (1997). The relationship between D2 receptor occupancy and plasma levels on low dose oral haloperidol: a PET study. Psychopharmacology (Berlin) 131: 148–152.
Kapur S, Zipursky RB, Remington G, Jones C, DaSilva J, Wilson AA et al (1998). 5-HT2 and D2 receptor occupancy of olanzapine in schizophrenia: a PET investigation. Am J Psychiatry 155: 921–928.
Kay SR, Fiszbein A, Opler LA (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13: 261–276.
Kessler R, Ansar AS, Lui R, Dawant B, Meltzer H (2003). Occupancy of cortical and substantia Nigra DA D2 receptors by typical and atypical antipsychotic drugs. Schizophr Res 60: 242–243.
Kessler RM, Whetsell WO, Ansari MS, Votaw JR, de Paulis T, Clanton JA et al (1993). Identification of extrastriatal dopamine D2 receptors in post mortem human brain with [125I]epidepride. Brain Res 609: 237–243.
Lammertsma AA, Hume SP (1996). Simplified reference tissue model for PET receptor studies. Neuroimage 4 (Part 1): 153–158.
Leucht S, Busch R, Hamann J, Kissling W, Kane JM (2005). Early-onset hypothesis of antipsychotic drug action: a hypothesis tested, confirmed and extended. Biol Psychiatry 57: 1543–1549.
Mogenson GJ, Jones DL, Yim CY (1980). From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol 14: 69–97.
Nordstrom AL, Farde L, Wiesel FA, Forslund K, Pauli S, Halldin C et al (1993). Central D2-dopamine receptor occupancy in relation to antipsychotic drug effects: a double-blind PET study of schizophrenic patients. Biol Psychiatry 33: 227–235.
Nyberg S, Farde L, Halldin C, Dahl ML, Bertilsson L (1995). D2 dopamine receptor occupancy during low-dose treatment with haloperidol decanoate. Am J Psychiatry 152: 173–178.
Nyberg S, Olsson H, Nilsson U, Maehlum E, Halldin C, Farde L (2002). Low striatal and extra-striatal D2 receptor occupancy during treatment with the atypical antipsychotic sertindole. Psychopharmacology (Berlin) 162: 37–41.
Olsson H, Halldin C, Farde L (2004). Differentiation of extrastriatal dopamine D2 receptor density and affinity in the human brain using PET. Neuroimage 22: 794–803.
Olsson H, Halldin C, Swahn CG, Farde L (1999). Quantification of [11C]FLB 457 binding to extrastriatal dopamine receptors in the human brain. J Cereb Blood Flow Metab 19: 1164–1173.
Pilowsky LS (2001). Probing targets for antipsychotic drug action with PET and SPET receptor imaging. Nucl Med Commun 22: 829–833.
Roberts RC, Knickman JK (2002). The ultrastructural organization of the patch matrix compartments in the human striatum. J Comp Neurol 452: 128–138.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E et al (1998). The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59 (Suppl 20): 22–33; quiz 34–57.
Simpson GM, Angus JW (1970). A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 212: 11–19.
Studholme C, Hill DL, Hawkes DJ (1997). Automated three-dimensional registration of magnetic resonance and positron emission tomography brain images by multiresolution optimization of voxel similarity measures. Med Phys 24: 25–35.
Talvik M, Nordstrom AL, Nyberg S, Olsson H, Halldin C, Farde L (2001). No support for regional selectivity in clozapine-treated patients: a PET study with [(11)C]raclopride and [(11)C]FLB 457. Am J Psychiatry 158: 926–930.
Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM (2004). Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci 27: 468–474.
Xiberas X, Martinot JL, Mallet L, Artiges E, Canal M, Loc HC et al (2001a). In vivo extrastriatal and striatal D2 dopamine receptor blockade by amisulpride in schizophrenia. J Clin Psychopharmacol 21: 207–214.
Xiberas X, Martinot JL, Mallet L, Artiges E, Loc HC, Maziere B et al (2001b). Extrastriatal and striatal D(2) dopamine receptor blockade with haloperidol or new antipsychotic drugs in patients with schizophrenia. Br J Psychiatry 179: 503–508.
Acknowledgements
We thank the staff of the PET Centre, especially Mr Armando Garcia for his expert assistance in radiochemistry, Mr Doug Hussey and Alvina NG for their expert technical assistance with scanning. We thank Ms Penny Barsoum for coordinating aspects of the study. This research is supported, in part, by the Canada Research Chair to Shitij Kapur and the Tapscott Chair in Schizophrenia Studies at the University of Toronto to Dr Robert B Zipursky. OA is supported by a NARSAD Young Investigator Award.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Agid, O., Mamo, D., Ginovart, N. et al. Striatal Vs Extrastriatal Dopamine D2 Receptors in Antipsychotic Response—A Double-Blind PET Study in Schizophrenia. Neuropsychopharmacol 32, 1209–1215 (2007). https://doi.org/10.1038/sj.npp.1301242
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1301242
Keywords
This article is cited by
-
Activation of the motivation-related ventral striatum during delusional experience
Translational Psychiatry (2018)
-
The relationship between excitement symptom severity and extrastriatal dopamine D2/3 receptor availability in patients with schizophrenia: a high-resolution PET study with [18F]fallypride
European Archives of Psychiatry and Clinical Neuroscience (2018)
-
Presynaptic Dopamine Synthesis Capacity in Schizophrenia and Striatal Blood Flow Change During Antipsychotic Treatment and Medication-Free Conditions
Neuropsychopharmacology (2017)
-
In vivo imaging of brain microglial activity in antipsychotic-free and medicated schizophrenia: a [11C](R)-PK11195 positron emission tomography study
Molecular Psychiatry (2016)
-
An investigation into aripiprazole’s partial D2 agonist effects within the dorsolateral prefrontal cortex during working memory in healthy volunteers
Psychopharmacology (2016)