Abstract
Human embryonic stem cells (hESCs) can proliferate indefinitely yet also differentiate in vitro, allowing normal human neurons to be generated in unlimited numbers. Here, we describe the development of an in vitro neurotoxicity assay using human dopaminergic neurons derived from hESCs. We showed that the dopaminergic neurotoxin 1-methyl-4-phenylpyridinium (MPP+), which produces features of Parkinson's disease in humans, was toxic for hESC-derived dopaminergic neurons. Treatment with glial cell line-derived neurotrophic factor protected tyrosine hydroxylase-positive neurons against MPP+-induced apoptotic cell death and loss of neuronal processes as well as against the formation of intracellular reactive oxygen species. The availability of human dopaminergic neurons, derived from hESCs, therefore allows for the possibility of directly examining the unique features of human dopaminergic neurons with respect to their responses to pharmacological agents as well as environmental and chemical toxins.
Similar content being viewed by others
INTRODUCTION
In vitro models provide important tools for the investigation of molecular and biochemical mechanisms involved in toxic processes, cellular differentiation, and developmental regulation. Because cells can be readily manipulated in vitro, cell lines and other cell preparations can be used in studies that are difficult or impossible to perform in intact animals including human subjects. The utility of in vitro models is, nevertheless, related to the accurate representation of the in vivo systems that they are purported to mimic. Often, because of convenience, availability, and consistency between preparations, immortal cell lines rather than primary cell cultures are employed for in vitro toxicity studies. There are, however, few cell lines that can serve as accurate in vitro models of neurons, and especially human central nervous system (CNS) neurons.
Because normal mature neurons do not generally divide and are thus not readily maintained in vitro, human neurons present great challenges for the development of adequate in vitro model systems. These difficulties have led to the use of substitute cell types such as tumor cells that express neuronal properties. Examples of them include PC12 cells, which were derived from a pheochromocytoma (Greene and Tischler, 1976), and SH-SY5Y cells, which were generated from a human neuroblastoma (Biedler et al, 1973). Ntera2, a human teratocarcinoma cell line that is readily maintained in vitro and can be differentiated into CNS neuronal phenotypes including dopaminergic neurons (Misiuta et al, 2003; Schwartz et al, 2005), has also been used. Nonetheless, these cell types have genetic differences from normal cells, and physiologically may diverge from normal cells in various respects. Primary cell cultures that more accurately represent normal neurons can be inconsistent from batch to batch. In addition, primary cultures of human neurons, and particularly human neurons with specific phenotypes such as dopaminergic neurons, are extremely difficult to obtain, as fetal tissue must be used.
The availability of human embryonic stem cells (hESCs), and methods for in vitro differentiation of neurons, including dopaminergic neurons, from hESCs allows for the possibility of generating human CNS neurons with essentially normal properties in unlimited quantities in vitro (Buytaert-Hoefen et al, 2004; Perrier et al, 2004; Thomson et al, 1998; Yan et al, 2005; Zeng et al, 2004a). This has the potential to lead to in vitro models with much greater accuracy for representation of human CNS neurons, and thus for the investigation of issues such as neuroprotection. Neurotoxicity and neuroprotective strategies are particularly important with respect to Parkinson's disease (PD), a neurodegenerative disease caused by the selective loss of dopaminergic neurons in the midbrain. Epidemiological data and twin studies suggest that environmental toxins may be important contributors to PD (Di Monte et al, 2002; Di Monte, 2003; Tanner et al, 1999). The dopaminergic neurons of the brain seem to be especially vulnerable to toxic events, as evidenced by their susceptibility to toxic insults, including rotenone, paraquat, and manganese (Betarbet et al, 2000; Thiruchelvam et al, 2000), lipopolysaccharides (Carvey et al, 2003; Gayle et al, 2002), diesel exhaust particles (Block et al, 2004) and N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (Davis et al, 1979; Langston et al, 1983). Therefore, an in vitro system for studies of neurotoxicity and neuroprotection in normal human dopaminergic neurons may be especially valuable.
In the present study, we developed an in vitro hESC model to study the neurotoxicity of 1-methyl-4-phenylpyridinium (MPP+), an oxidative metabolite of neurotoxin MPTP, which is specific for dopaminergic neurons. This hESC-based model of MPP+-induced neurotoxicity was then used to examine whether human dopaminergic neurons can be protected from damage by the glial cell line-derived neurotrophic factor (GDNF).
MATERIALS AND METHODS
hESC Culture and Dopaminergic Differentiation
hESC lines (BG01 and I6) were either maintained on inactivated mouse embryonic fibroblast (MEF) feeder cells in Dulbecco's modified Eagle's medium/Ham's F12 (DMEM/F12, 1 : 1) supplemented with 20% knockout serum replacement (KSR), 2 mM nonessential amino acids, 2 mM L-glutamine, 50 μg/ml Penn-Strep (all from Invitrogen, Carlsbad, CA), 0.1 mM β-mercaptoethanol (Specialty Media, Phillipsburg, NJ), and 4 ng/ml of basic fibroblast growth factor (bFGF, Sigma, St Louis, MO), or on fibronectin-coated dishes in medium conditioned with MEF for 24 h as described previously (Brimble et al, 2004). Cells were passaged either manually or enzymatically every 5 days. The medium was changed every day.
Dopaminergic differentiation of hESCs was induced by the mouse stromal cell line PA6 as described previously (Zeng et al, 2004a, Zeng et al, 2004b). Briefly, hESCs were seeded at a density of approximately 1000 clumps/3 cm dish on a confluent layer of PA6 feeder cells in Glasgow Minimum Essential Media (GMEM, Invitrogen) supplemented with 10% KSR (Invitrogen), 1 mM pyruvate (Sigma), 0.1 mM nonessential amino acids, and 0.1 mM β-mercaptoethanol. hESCs were allowed to differentiate into dopaminergic neurons for 3 weeks on PA6 cells before drug treatment. A colony was counted as a TH+ colony if at least 25 individual cells in the colony were stained for tyrosine hydroxylase (TH). Microscopic fields were randomly chosen for counting and statistical analysis.
MPP+ and GDNF Treatments
MPP+ (Sigma) was dissolved in ultrapure sterile H2O and was freshly prepared for each experiment. In most of the experiments, hESCs that had been cocultured with PA6 cells for 3 weeks were exposed to 1 mM MPP+ for 24 h, except for time course and dosage response studies, where the concentration of MPP+ and the drug exposure time are specifically indicated. For GDNF neuroprotective experiments, unless specifically stated, 25 ng/ml of GDNF (R&D Systems, Minneapolis, MN) was added to cultures 1 h before MPP+ treatment.
Immunocytochemistry
Expression of TH was examined by immunocytochemistry using staining procedures described previously (Zeng et al, 2003). Briefly, hESCs differentiated on PA6 cells were fixed with 2% paraformaldehyde for 30 min, blocked in blocking buffer (5% goat serum, 1% BSA, 0.1% Triton X-100) for 1 h, and incubated in the primary antibody (1 : 1000, Pel Freez, Rogers, AR) at 4°C overnight. Appropriately coupled secondary antibody (Molecular Probes) was tested for crossreactivity and nonspecific immunoreactivity. 4′,6-Diamidino-2-phenylindole (DAPI, 1 : 1000, Sigma) was used to identify nuclei. Images were captured on an Olympus fluorescence microscope.
For cleaved caspase-3 immunohistochemistry, 24 h after MPP+ treatment, the cells were treated with 0.3% Triton X-100 for 10 min at room temperature, fixed with 4% paraformaldehyde for 30 min at 4°C, followed by overnight incubation with polyclonal cleaved caspase-3 primary antibody (Cell Signaling, Beverly, MA). Subsequent processing with biotinylated secondary antibody and ABC complex was performed according to the manufacturer's procedures as described in the ABC kit (Vector Laboratories, Burlingame, CA). The cells were then reacted with 3,3′-diaminobenzidine and hydrogen peroxide to visualize the peroxidase reaction. Positive cells were counted under a × 20 objective lens in 10 randomly selected photographic fields for each group for statistical analysis. Images were taken using a Zeiss LSC System with an Axiovert 135 microscope.
Measurement of Lactate Dehydrogenase (LDH) Activity
General cytotoxicity was determined by the release of LDH into the culture medium, using a nonradioactive cytotoxicity assay kit (Promega, Madison, WI) according to the manufacturer's protocol. Absorbance at 490 nm is owing to the formation of nicotinamide adenine dinucleotide, which results in the conversion of a tetrazolium (INT) into a red formazan product. The amount of color formed is proportional to the number of lysed cells. Data are expressed as percentages of the control samples.
Measurement of Intracellular Reactive Oxygen Species (ROS) Formation
The dye DCFH2-DA, which is oxidized to fluorescent DCF by hydroperoxides, was used to measure relative levels of cellular peroxides (Park et al, 2003). After exposure to MPP+, 100 μl of dye in ethanol was added to the culture media at a final concentration of 500 μM and incubated for 30 min at 37°C. To obtain dissociated hESCs for the ROS assay, cells were washed twice with phosphate-buffered saline (PBS) and then scraped from the dish with a pipet tip into 200 μl of 1% triton X-100 in PBS. Fifty microliters of cell suspension was measured in duplicate by a fluorescence microplate reader at an excitation wavelength of 485 nm and an emission wavelength of 530 nm. Hundred microliters of ethanol (without DCFH2-DA) was added to another cell sample to correct for autofluorescence generated by the cells. The same volume of 1% Triton X-100 buffer with DCFH2-DA without cells was set up as a blank control. Data are expressed as percentages of control fluorescence intensity units/mg protein. The experiment was repeated three times.
RESULTS
MPP+ Toxicity
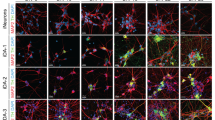
After 3 weeks of differentiation, approximately 80% of hESC colonies contained TH-positive neurons. At that time, more than 50% of colonies contained a high percentage (20–50%) of TH+ cells. A typical hESC culture after 3 weeks of differentiation is shown in Figure 1a (left panels), in which several differentiated colonies and numerous TH-positive cells with extensive process formation can be seen. Previous studies using the same differentiation protocol have shown that dopaminergic neurons derived from hESCs express many markers of dopaminergic neurons, are postmitotic (Zeng et al, 2004a), and are electrophysiologically active (unpublished data).
MPP+ toxicity for hESC-derived dopaminergic neurons is evidenced by decreased numbers of TH-positive cells, loss of processes, release of LDH, and generation of ROS. (a) Immunocytochemical analysis by TH showed a significant decrease in TH-positive cells after 1 mM MPP+ for 24 h, and the surviving TH-positive had an immature appearance with an apparent loss of processes. Scale bar: 400 μm for × 5 and 100 μm for × 20. Inset: An example of TH-positive cells in a colony stained with DAPI to show cell nuclei. (b) The decrease in TH-positive colonies was dose-related, with a three-fold decrease for the highest concentration of MPP+ (5 mM). (c) LDH was released into the culture medium following MPP+. LDH activity was increased approximately two-fold 24 h after 1 mM MPP+. (d) ROS generation was increased after MPP+ treatment. *p<0.05, **p<0.01 and ***p<0.001; Tukey compromise following significant one-way ANOVA.
After treatment with 1 mM MPP+ for 24 h, there was a substantial decrease in TH-positive colonies and TH-positive cells within colonies (Figure 1a and b). In addition, there was an apparent loss of processes by the TH-positive cells, and the remaining surviving TH-positive cells had an immature appearance (Figure 1a, right panel). The number of colonies containing TH-positive cells showed a dose-related decrease, from 79±2.4% in control cells to 26±3.9% in cells treated with 5 mM MPP+ (Figure 1b). Additional loss of TH-positive colonies occurred between 24 and 48 h for lower concentrations of MPP+ (0.5 and 1 mM), but not for the highest concentration (5 mM) (Supplementary Figure 1a), indicating that the toxic effect was already maximal by 24 h for 5 mM MPP+. MPP+ caused a dose-related toxic effect at 24 h as measured by the release of LDH into the medium (Figure 1c). After 48 h, there was an additional increase in LDH for the lower doses (0.5 and 1 mM), but no further increase for the highest dose (5 mM) of MPP+ (Supplementary Figure 1b). Therefore, the LDH data are consistent with the results for TH+ cell loss. LDH release was not induced by MPP+ treatment of either undifferentiated hESCs or PA6 cells.
ROS Production
As MPP+ neurotoxicity is believed to be mediated, at least partially, by ROS generation, we monitored ROS production during MPP+ treatment using the DCF fluorescence assay for intracellular peroxides. As shown in Figure 1d, MPP+ produced a dose-dependent increase in ROS at 24 h, with a substantial increase even for the lowest concentration (0.5 mM) of MPP+ tested. By 48 h, ROS had decreased nearly to control levels (data not shown). MPP+ treatment of undifferentiated hESCs or PA6 cells did not increase ROS production (data not shown).
Neuroprotective Effects of GDNF
GDNF has been demonstrated to exert a neuroprotective effect in rodent and subhuman primate PD models both in vivo and in vitro. We therefore tested whether GDNF is able to protect hESC-derived dopaminergic neurons from MPP+ toxicity. As high concentrations of GDNF can have a toxic effect on neurons, we tested the dosage response to GDNF in our culture system. By TH immunofluorescence analysis, the number of TH-positive colonies and the morphology of TH-positive cells in samples treated with 25 ng/ml GDNF for 24 h were similar to untreated control cells (Figure 2a). Higher concentrations of GDNF (>50 ng/ml) produced a toxic effect (data not shown).
GDNF protected human dopaminergic neurons from MPP+ toxicity. (a) Immunocytochemical analysis for TH revealed that pretreatment with GDNF led to a marked preservation of TH-positive cells as well as a preservation of TH-positive processes. (b) TH-positive colony counts showed that GDNF almost entirely prevented the loss of TH-positive colonies. By a two-way ANOVA, the main effects of GDNF and MPP+, and the interaction, were statistically significant (all p<0.0001). (c) LDH was reduced by approximately 30%, when cells were pretreated with 25 ng/ml GDNF. By a two-way ANOVA, the main effect of MPP+ was statistically significant (p<0.0001), as was the interaction (p=0.012). The main effect of GDNF was marginal (p=0.054). (d) ROS levels were restored nearly to control values in samples pretreated with 25 ng/ml GDNF. By a two-way ANOVA, the main effect of GDNF was statistically significant (p=0.021), but the main effect of MPP+ was not (p=0.17). The interaction was significant (p<0.001). ***p<0.001, and ****p<0.0001 as compared to control; ++p<0.01 and ++++p<0.0001 as compared to MPP+ only. Scale bar: 400 μm for × 5 and 100 μm for × 20.
Although MPP+ caused cell shrinkage and processes retraction (Figure 2a), pretreatment with GDNF led to marked preservation of TH-positive cells as well as maintenance of TH-positive processes (Figure 2a). TH-positive colony counts revealed that GDNF almost entirely prevented the toxicity of MPP+: 69±1.2% of colonies were TH-positive in cultures pretreated with GDNF before MPP+, as compared to 77±2.3% in control cultures and 75±2.2% in cultures treated with GDNF only (Figure 2b). In contrast, only 41±2.1% of colonies treated with MPP+ were positive for TH. In addition, GDNF protected against MPP+ toxicity as measured by LDH release (Figure 2c).
We also investigated the possibility that pretreatment with GDNF has the capacity to prevent ROS accumulation in dopaminergic neurons following MPP+ treatment. No increase in ROS was observed after treatment with GDNF alone, whereas ROS was elevated after MPP+ (Figure 2d). Pretreatment with GDNF before MPP+ decreased ROS to control levels (Figure 2d). Therefore, GDNF does not simply protect human dopaminergic neurons from the consequences of ROS accumulation, but also prevents accumulation of ROS per se.
MPP+-Induced Cell Death via Apoptosis
Because it is generally believed that apoptosis contributes to MPP+ neurotoxicity and is a possible causative factor in the development of PD in humans, we used immunocytochemistry for active caspase to determine the nature of cell death caused by MPP+. Only a few caspase-3-immunoreactive cells (∼20 cells/field) were observed in the control and GDNF-treated samples, whereas a significantly stronger signal and larger numbers of caspase-3-positive cells (∼230 cells/field) were found in the MPP+-treated group (Figure 3a and b). The increase in caspase-3-positive cells following MPP+ treatment was significantly attenuated by pretreatment with GDNF (Figure 3a). Morphologically, a few well-differentiated, fine-process bearing cells were found to be positive, whereas most caspase-3-positive signals were easily discerned as cellular nuclei (Figure 3).
GDNF prevents MPP+-induced activation of caspase-3. (a) A few caspase-3-positive cells were observed in control and GDNF-treated dishes, whereas many more positive cells were present after MPP+ treatment. Caspase-3 activation was attenuated by GDNF application. (b) Quantification of caspase-3-positive after MPP+ and GDNF treatments. **p<0.01 as compared to control and ++p<0.01 as compared to MPP+ only; Tukey compromise following ANOVA. Scale bar=100 μm.
DISCUSSION
The main pathological hallmark of PD is a progressive loss of substantia nigra dopaminergic neurons in the midbrain. Understanding the mechanism of neuronal cell death involved in PD may be of value in developing neuroprotective therapies. Given the methodological and biological difficulties in studying neuronal cell death in human brains, in vitro models of dopaminergic cell death are powerful, as they allow the study of neurodegeneration as well as novel therapeutic strategies. Nevertheless, with very few exceptions (Clarkson et al, 1997), it has not been possible to examine directly the toxic effects of neurotoxins and protective effects of multiple factors for normal human dopaminergic neurons, because the availability of human dopaminergic neurons derived from fetal material is extremely limited. In the present study, we showed that hESCs can provide an unlimited source of normal human dopaminergic neurons for in vitro studies of neurotoxic and neuroprotective processes that might be related to PD.
In vitro models of MPP+-induced cell death are widely used because MPTP produces selective nigral neuronal death in many species including humans and subhuman primates, and because these abnormalities are associated with motor symptoms reminiscent of PD (Burns et al, 1983; Davis et al, 1979; Langston et al, 1983; Porrino et al, 1987). Because GDNF can exert neuroprotective effects against MPP+ toxicity in rodent and subhuman primate PD models, it has been considered to be one of the most promising strategies in attempts to delay the progression of the human disorder (Akerud et al, 2001; Gash et al, 1998; Granholm et al, 2000; Lin et al, 1993; Tomac et al, 1995; Zhang et al, 1997). The present data using human dopaminergic cells are consistent with data obtained from rodent and subhuman primate PD models. Using hESC-derived dopaminergic neurons, we showed that MPP+ causes dose-dependent cell death of TH-positive neurons by activating a caspase-3-dependent apoptotic pathway. This seems to occur via the production of ROS, as GDNF, which protects against MPP+ toxic effects, markedly attenuated ROS production and caspase-3 activation.
One of the major mechanisms of cell death in dopaminergic neurons is believed to involve ROS (Cadet and Brannock, 1998), and dopamine itself can lead to the production of ROS through a number of processes (Teismann et al, 2003). There have been studies that suggest that GDNF can protect dopaminergic neurons against the toxic sequelae of ROS. For example, vitamin D, which increases GDNF production, protects against damage of dopaminergic neurons induced by both 6-hydroxydopamine and H2O2 (Son et al, 1999; Wang et al, 2001). GDNF also has protective effects against the consequences of ROS damage for cochlear cells (Yamasoba et al, 1999). In cultured motor neurons, GDNF was found to inhibit both the formation of ROS and ROS-induced biochemical reactions (Irie and Hirabayashi, 1999). The present data suggest that the protective effect of GDNF against MPP+-induced damage of dopaminergic neurons is mediated at least in part by a reduction in ROS formation.
Although the mechanism of PA6 induction of dopaminergic differentiation remains unclear, it is believed that factors secreted by PA6 cells may be responsible for this effect. Yamazoe et al (2005) have recently showed that dopaminergic differentiation of mouse ESCs can be induced by medium collected by washing PA6 cells with PBS containing heparin. We have found that PA6 cell-conditioned medium can induce dopaminergic differentiation of an NCAM+ FACS-isolated NTera2 cell population (Schwartz et al, 2005). Thus, it is reasonable to ask whether MPP+ treatment could have an effect on the ability of the PA6 cells to secrete the appropriate conditioning factors, which might account for the decrease in TH+ cells by MPP+ treatment. We believe that this is unlikely, as we did not observe increased cell death as measured by LDH release, or increased ROS formation, in PA6 cells treated with the highest dose (5 mM) of MPP+. In addition, even if MPP+ had some other effect on PA6 cells, it would be unlikely to have an impact on these experiments because PA6 cells are not required for maintaining the dopaminergic phenotype at the time of MPP+ treatment (3 weeks postdifferentiation). This is supported by the fact that hESCs continue to differentiate into dopaminergic neurons in the absence of stromal cells when passaged after coculturing with stromal cells for 1–3 weeks (Perrier et al, 2004). Nevertheless, the possibility that either MPP+ or GDNP causes a change in PA6 cell function cannot be entirely ruled out.
There are marked species differences in the sensitivity to MPTP and its active metabolite, MPP+ (Kalaria and Harik, 1986; Kalaria et al, 1987). Rats are almost entirely insensitive to MPP+, mice are intermediately sensitive, whereas non-human primates are highly sensitive to MPP+ and MPTP toxicity. Based on the Parkinsonism observed in human subjects that have been exposed to MPTP, humans are believed to be highly sensitive as well, although this cannot be tested directly. The reasons for these differences are not entirely understood. One major factor appears to be monoamine oxidase activity in brain microvessels (Kalaria and Harik, 1987; Riachi and Harik, 1988). It is believed that the high monoamine oxidase activity in rodent microvessels results in metabolism of MPTP within blood vessels, thus obstructing the entry of MPTP into the brain (Riachi and Harik, 1988). In addition, there are differences in metabolism of MPTP in rodents as compared to primates, which may also contribute to higher and more prolonged concentrations of MPP+ in the primate brain (Giovanni et al, 1994). There is also, however, a 10-fold difference between rats and mice in susceptibility to MPP+ when administered directly into the striatum, so at least some of the differences between species seem to be unrelated to the conversion of MPTP to MPP+ (Johannessen et al, 1985). Differences in vesicular storage capacity for MPP+, possibly owing to differences in the vesicular monoamine transporter itself (Lesch et al, 1993; Roussa and Krieglstein, 2004; Staal et al, 2000), as well as differences in the dopamine transporter (Mitsuhata et al, 1998) have been suggested to contribute to the differential susceptibility. Another factor that may contribute to sensitivity of primates to MPP+ is binding to neuromelanin (D'Amato et al, 1987). Because of differences between human and subhuman dopaminergic neurons, an in vitro model of human dopaminergic neurons may be especially valuable. Human dopaminergic neurons may also be differentially sensitive to a variety of toxic agents, and the availability of human dopaminergic neurons derived from hESCs may thus facilitate the study of toxic mechanisms in PD in general, as well as mechanisms involved in other disorders, which may involve the brain dopaminergic systems such as schizophrenia and drug abuse.
In these initial studies using hESCs, we have shown that exposing hESC-derived dopaminergic neurons to MPP+ led to the death of these cells via a process that resembles apoptosis. In addition, treatment of these cells with GDNF afforded them protection against this well-known dopaminergic toxin. These observations suggest that hESC-based in vitro models might be helpful in studies of various cellular and molecular pathways that are involved in the normal function and degeneration of human dopaminergic neurons.
References
Akerud P, Canals JM, Snyder EY, Arenas E (2001). Neuroprotection through delivery of glial cell line-derived neurotrophic factor by neural stem cells in a mouse model of Parkinson's disease. J Neurosci 21: 8108–8118.
Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT (2000). Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci 3: 1301–1306.
Biedler JL, Helson L, Spengler BA (1973). Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res 33: 2643–2652.
Block ML, Wu X, Pei Z, Li G, Wang T, Qin L et al (2004). Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: the role of microglia, phagocytosis, and NADPH oxidase. FASEB J 18: 1618–1620.
Brimble SN, Zeng X, Weiler DA, Luo Y, Liu Y, Lyons IG et al (2004). Karyotypic stability, genotyping, differentiation, feeder-free maintenance, and gene expression sampling in three human embryonic stem cell lines derived prior to August 9, 2001. Stem Cells Dev 13: 585–597.
Burns RS, Chiueh CC, Markey SP, Ebert MH, Jacobowitz DM, Kopin IJ (1983). A primate model of Parkinsonism-selective destruction of dopaminergic-neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci USA 80: 4546–4550.
Buytaert-Hoefen KA, Alvarez E, Freed CR (2004). Generation of tyrosine hydroxylase positive neurons from human embryonic stem cells after coculture with cellular substrates and exposure to GDNF. Stem Cells 22: 669–674.
Cadet JL, Brannock C (1998). Free radicals and the pathobiology of brain dopamine systems. Neurochem Int 32: 117–131.
Carvey PM, Chang Q, Lipton JW, Ling Z (2003). Prenatal exposure to the bacteriotoxin lipopolysaccharide leads to long-term losses of dopamine neurons in offspring: a potential, new model of Parkinson's disease. Front Biosci 8: S826–S837.
Clarkson ED, Zawada WM, Freed CR (1997). GDNF improves survival and reduces apoptosis in human embryonic dopaminergic neurons in vitro. Cell Tissue Res 289: 207–210.
D'Amato RJ, Alexander GM, Schwartzman RJ, Kitt CA, Price DL, Snyder SH (1987). Evidence for neuromelanin involvement in MPTP-induced neurotoxicity. Nature 327: 324–326.
Davis GC, Williams AC, Markey SP, Ebert MH, Caine ED, Reichert CM et al (1979). Chronic Parkinsonism secondary to intravenous injection of meperidine analogues. Psychiatry Res 1: 249–254.
Di Monte DA (2003). The environment and Parkinson's disease: is the nigrostriatal system preferentially targeted by neurotoxins? Lancet Neurol 2: 531–538.
Di Monte DA, Lavasani M, Manning-Bog AB (2002). Environmental factors in Parkinson's disease. Neurotoxicology 23: 487–502.
Gash DM, Gerhardt GA, Hoffer BJ (1998). Effects of glial cell line-derived neurotrophic factor on the nigrostriatal dopamine system in rodents and non-human primates. Adv Pharmacol 42: 911–915.
Gayle DA, Ling Z, Tong C, Landers T, Lipton JW, Carvey PM (2002). Lipopolysaccharide (LPS)-induced dopamine cell loss in culture: roles of tumor necrosis factor-alpha, interleukin-1beta, and nitric oxide. Brain Res Dev Brain Res 133: 27–35.
Giovanni A, Sonsalla PK, Heikkila RE (1994). Studies on species sensitivity to the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Part 2: central administration of 1-methyl-4-phenylpyridinium. J Pharmacol Exp Ther 270: 1008–1014.
Granholm AC, Reyland M, Albeck D, Sanders L, Gerhardt G, Hoernig G et al (2000). Glial cell line-derived neurotrophic factor is essential for postnatal survival of midbrain dopamine neurons. J Neurosci 20: 3182–3190.
Greene LA, Tischler AS (1976). Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA 73: 2424–2428.
Irie F, Hirabayashi Y (1999). Ceramide prevents motoneuronal cell death through inhibition of oxidative signal. Neurosci Res 35: 135–144.
Johannessen JN, Chiueh CC, Burns RS, Markey SP (1985). Differences in the metabolism of MPTP in the rodent and primate parallel differences in sensitivity to its neurotoxic effects. Life Sci 36: 219–224.
Kalaria RN, Harik SI (1986). Nucleoside transporter of cerebral microvessels and choroid plexus. J Neurochem 47: 1849–1856.
Kalaria RN, Harik SI (1987). Blood–brain barrier monoamine oxidase: enzyme characterization in cerebral microvessels and other tissues from six mammaliam species, including human. J Neurochem 49: 856–864.
Kalaria RN, Mitchell MJ, Harik SI (1987). Correlation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity with blood–brain barrier monoamine oxidase activity. Proc Natl Acad Sci USA 84: 3521–3525.
Langston JW, Ballard P, Tetrud JW, Irwin I (1983). Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219: 979–980.
Lesch KP, Gross J, Wolozin BL, Murphy DL, Riederer P (1993). Extensive sequence divergence between the human and rat brain vesicular monoamine transporter: possible molecular basis for species differences in the susceptibility to MPP+. J Neural Transm Gen Sect 93: 75–82.
Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F (1993). GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 260: 1130–1132.
Misiuta IE, Anderson L, McGrogan MP, Sanberg PR, Willing AE, Zigova T (2003). The transcription factor Nurr1 in human NT2 cells and hNT neurons. Brain Res Mol Brain Res 145: 107–115.
Mitsuhata C, Kitayama S, Morita K, Vandenbergh D, Uhl GR, Dohi T (1998). Tyrosine-533 of rat dopamine transporter: involvement in interactions with 1-methyl-4-phenylpyridinium and cocaine. Brain Res Mol Brain Res 56: 84–88.
Park TH, Kwon OS, Park SY, Han ES, Lee CS (2003). N-methylated beta-carbolines protect PC12 cells from cytotoxic effect of MPP+ by attenuation of mitochondrial membrane permeability change. Neurosci Res 46: 349–358.
Perrier AL, Tabar V, Barberi T, Rubio ME, Bruses J, Topf N et al (2004). Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci USA 101: 12543–12548.
Porrino LJ, Burns RS, Crane AM, Palombo E, Kopin IJ, Sokoloff L (1987). Local cerebral metabolic effects of L-dopa therapy in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinsonism in monkeys. Proc Natl Acad Sci USA 84: 5995–5999.
Riachi NJ, Harik SI (1988). Strain differences in systemic 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity in mice correlate best with monoamine oxidase activity at the blood-brain barrier. Life Sci 42: 2359–2363.
Roussa E, Krieglstein K (2004). GDNF promotes neuronal differentiation and dopaminergic development of mouse mesencephalic neurospheres. Neurosci Lett 361: 52–55.
Schwartz CM, Spivak CE, Baker SC, McDaniel TK, Loring JF, Nguyen C et al (2005). NTera2: a model system to study dopaminergic differentiation of human embryonic stem cells. Stem Cells Dev 14: 517–534.
Son JH, Chun HS, Joh TH, Cho S, Conti B, Lee JW (1999). Neuroprotection and neuronal differentiation studies using substantia nigra dopaminergic cells derived from transgenic mouse embryos. J Neurosci 19: 10–20.
Staal RG, Hogan KA, Liang CL, German DC, Sonsalla PK (2000). In vitro studies of striatal vesicles containing the vesicular monoamine transporter (VMAT2): rat versus mouse differences in sequestration of 1-methyl-4-phenylpyridinium. J Pharmacol Exp Ther 293: 329–335.
Tanner CM, Ottman R, Goldman SM, Ellenberg J, Chan P, Mayeux R et al (1999). Parkinson disease in twins: an etiologic study. JAMA 281: 341–346.
Teismann P, Tieu K, Cohen O, Choi DK, Wu DC, Marks D et al (2003). Pathogenic role of glial cells in Parkinson's disease. Mov Disord 18: 121–129.
Thiruchelvam M, Richfield EK, Baggs RB, Tank AW, Cory-Slechta DA (2000). The nigrostriatal dopaminergic system as a preferential target of repeated exposures to combined paraquat and maneb: implications for Parkinson's disease. J Neurosci 20: 9207–9214.
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS et al (1998). Embryonic stem cell lines derived from human blastocysts. Science 282: 1145–1147.
Tomac A, Lindqvist E, Lin LF, Ogren SO, Young D, Hoffer BJ et al (1995). Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature 373: 335–339.
Wang JY, Wu JN, Cherng TL, Hoffer BJ, Chen HH, Borlongan CV et al (2001). Vitamin D(3) attenuates 6-hydroxydopamine-induced neurotoxicity in rats. Brain Res 904: 67–75.
Yamasoba T, Schacht J, Shoji F, Miller JM (1999). Attenuation of cochlear damage from noise trauma by an iron chelator, a free radical scavenger and glial cell line-derived neurotrophic factor in vivo. Brain Res 815: 317–325.
Yamazoe H, Murakami Y, Mizuseki K, Sasai Y, Iwata H (2005). Collection of neural inducing factors from PA6 cells using heparin solution and their immobilization on plastic culture dishes for the induction of neurons from embryonic stem cells. Biomaterials 26: 5746–5754.
Yan Y, Yang D, Zarnowska ED, Du Z, Werbel B, Valliere C et al (2005). Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells 23: 781–790.
Zeng X, Cai J, Chen J, Luo Y, You ZB, Fotter E et al (2004a). Dopaminergic differentiation of human embryonic stem cells. Stem Cells 22: 925–940.
Zeng X, Chen J, Sanchez JF, Coggiano M, Dillon-Carter O, Petersen J et al (2003). Stable expression of hrGFP by mouse embryonic stem cells: promoter activity in the undifferentiated state and during dopaminergic neural differentiation. Stem Cells 21: 647–653.
Zeng X, Miura T, Luo Y, Bhattacharya B, Condie B, Chen J et al (2004b). Properties of pluripotent human embryonic stem cells BG01 and BG02. Stem Cells 22: 292–312.
Zhang Z, Miyoshi Y, Lapchak PA, Collins F, Hilt D, Lebel C et al (1997). Dose response to intraventricular glial cell line-derived neurotrophic factor administration in parkinsonian monkeys. J Pharmacol Exp Ther 282: 1396–1401.
Acknowledgements
This research was supported by the IRP of NIDA, NIDA, and DHHS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary information
Rights and permissions
About this article
Cite this article
Zeng, X., Chen, J., Deng, X. et al. An In Vitro Model of Human Dopaminergic Neurons Derived from Embryonic Stem Cells: MPP+ Toxicity and GDNF Neuroprotection. Neuropsychopharmacol 31, 2708–2715 (2006). https://doi.org/10.1038/sj.npp.1301125
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1301125
Keywords
This article is cited by
-
Corynoxine B derivative CB6 prevents Parkinsonian toxicity in mice by inducing PIK3C3 complex-dependent autophagy
Acta Pharmacologica Sinica (2022)
-
In vitro acute and developmental neurotoxicity screening: an overview of cellular platforms and high-throughput technical possibilities
Archives of Toxicology (2017)
-
CYP3A5 Mediates Effects of Cocaine on Human Neocorticogenesis: Studies using an In Vitro 3D Self-Organized hPSC Model with a Single Cortex-Like Unit
Neuropsychopharmacology (2017)
-
Current Neurogenic and Neuroprotective Strategies to Prevent and Treat Neurodegenerative and Neuropsychiatric Disorders
NeuroMolecular Medicine (2015)
-
Present state and future perspectives of using pluripotent stem cells in toxicology research
Archives of Toxicology (2011)