Abstract
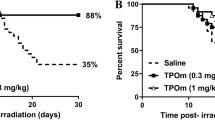
Tissue damage induced by ionizing radiation in the hematopoietic and gastrointestinal systems is the major cause of lethality in radiological emergency scenarios and underlies some deleterious side effects in patients undergoing radiation therapy1,2. The identification of target-specific interventions that confer radiomitigating activity is an unmet challenge. Here we identify the thrombomodulin (Thbd)–activated protein C (aPC) pathway as a new mechanism for the mitigation of total body irradiation (TBI)-induced mortality. Although the effects of the endogenous Thbd-aPC pathway were largely confined to the local microenvironment of Thbd-expressing cells, systemic administration of soluble Thbd or aPC could reproduce and augment the radioprotective effect of the endogenous Thbd-aPC pathway. Therapeutic administration of recombinant, soluble Thbd or aPC to lethally irradiated wild-type mice resulted in an accelerated recovery of hematopoietic progenitor activity in bone marrow and a mitigation of lethal TBI. Starting infusion of aPC as late as 24 h after exposure to radiation was sufficient to mitigate radiation-induced mortality in these mice. These findings suggest that pharmacologic augmentation of the activity of the Thbd-aPC pathway by recombinant Thbd or aPC might offer a rational approach to the mitigation of tissue injury and lethality caused by ionizing radiation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mauch, P. et al. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 31, 1319–1339 (1995).
Kirsch, D.G. et al. p53 controls radiation-induced gastrointestinal syndrome in mice independent of apoptosis. Science 327, 593–596 (2010).
Schuller, B.W. et al. Selective irradiation of the vascular endothelium has no effect on the survival of murine intestinal crypt stem cells. Proc. Natl. Acad. Sci. USA 103, 3787–3792 (2006).
Rotolo, J.A. et al. Regulation of ceramide synthase-mediated crypt epithelium apoptosis by DNA damage repair enzymes. Cancer Res. 70, 957–967 (2010).
Paris, F. et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science 293, 293–297 (2001).
Kustikova, O.S. et al. Retroviral vector insertion sites associated with dominant hematopoietic clones mark “stemness” pathways. Blood 109, 1897–1907 (2007).
Yu, H. et al. Deletion of Puma protects hematopoietic stem cells and confers long-term survival in response to high-dose gamma-irradiation. Blood 115, 3472–3480 (2010).
Kanzawa, T. et al. Ionizing radiation induces apoptosis and inhibits neuronal differentiation in rat neural stem cells via the c-Jun NH2-terminal kinase (JNK) pathway. Oncogene 25, 3638–3648 (2006).
Weiler, H. & Isermann, B.H. Thrombomodulin. J. Thromb. Haemost. 1, 1515–1524 (2003).
Wang, W., Nagashima, M., Schneider, M., Morser, J. & Nesheim, M. Elements of the primary structure of thrombomodulin required for efficient thrombin-activable fibrinolysis inhibitor activation. J. Biol. Chem. 275, 22942–22947 (2000).
Esmon, N.L., Owen, W.G. & Esmon, C.T. Isolation of a membrane-bound cofactor for thrombin-catalyzed activation of protein C. J. Biol. Chem. 257, 859–864 (1982).
Esmon, C.T., Esmon, N.L. & Harris, K.W. Complex formation between thrombin and thrombomodulin inhibits both thrombin-catalyzed fibrin formation and factor V activation. J. Biol. Chem. 257, 7944–7947 (1982).
Mosnier, L.O., Zlokovic, B.V. & Griffin, J.H. The cytoprotective protein C pathway. Blood 109, 3161–3172 (2007).
Weiler, H. Regulation of inflammation by the protein C system. Crit. Care Med. 38, S18–S25 (2010).
Tanaka, K.A. et al. Soluble thrombomodulin is antithrombotic in the presence of neutralising antibodies to protein C and reduces circulating activated protein C levels in primates. Br. J. Haematol. 132, 197–203 (2006).
van Iersel, T., Stroissnig, H., Giesen, P., Wemer, J. & Wilhelm-Ogunbiyi, K. Phase I study of Solulin, a novel recombinant soluble human thrombomodulin analogue. Thromb. Haemost. 105, 302–312 (2011).
Su, E.J. et al. The thrombomodulin analog Solulin promotes reperfusion and reduces infarct volume in a thrombotic stroke model. J. Thromb. Haemost. 9, 1174–1182 (2011).
Foley, J.H., Rea, C.J., Petersen, K.U., Nesheim, M.E. & Sorensen, B. Solulin increases clot stability in whole blood from humans and dogs with hemophilia. Blood 119, 3622–3628 (2012).
Mosnier, L.O., Yang, X.V. & Griffin, J.H. Activated protein C mutant with minimal anticoagulant activity, normal cytoprotective activity, and preservation of thrombin activable fibrinolysis inhibitor-dependent cytoprotective functions. J. Biol. Chem. 282, 33022–33033 (2007).
Kerschen, E. et al. Activated protein C targets CD8+ dendritic cells to reduce the mortality of endotoxemia in mice. J. Clin. Invest. 120, 3167–3178 (2010).
Mosnier, L.O. et al. Hyperantithrombotic, noncytoprotective Glu149Ala-activated protein C mutant. Blood 113, 5970–5978 (2009).
Ramalho-Santos, M., Yoon, S., Matsuzaki, Y., Mulligan, R.C. & Melton, D.A. “Stemness”: transcriptional profiling of embryonic and adult stem cells. Science 298, 597–600 (2002).
Kent, D.G. et al. Prospective isolation and molecular characterization of hematopoietic stem cells with durable self-renewal potential. Blood 113, 6342–6350 (2009).
Balazs, A.B., Fabian, A.J., Esmon, C.T. & Mulligan, R.C. Endothelial protein C receptor (CD201) explicitly identifies hematopoietic stem cells in murine bone marrow. Blood 107, 2317–2321 (2006).
Iwasaki, H., Arai, F., Kubota, Y., Dahl, M. & Suda, T. Endothelial protein C receptor-expressing hematopoietic stem cells reside in the perisinusoidal niche in fetal liver. Blood 116, 544–553 (2010).
Darrow, A.L. et al. Biological consequences of thrombin receptor deficiency in mice. Thromb. Haemost. 76, 860–866 (1996).
Iwaki, T., Cruz, D.T., Martin, J.A. & Castellino, F.J. A cardioprotective role for the endothelial protein C receptor in lipopolysaccharide-induced endotoxemia in the mouse. Blood 105, 2364–2371 (2005).
Xu, J. et al. Extracellular histones are major mediators of death in sepsis. Nat. Med. 15, 1318–1321 (2009).
Xu, J., Zhang, X., Monestier, M., Esmon, N.L. & Esmon, C.T. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J. Immunol. 187, 2626–2631 (2011).
Luo, D. et al. Factor XI–deficient mice display reduced inflammation, coagulopathy, and bacterial growth during listeriosis. Infect. Immun. 80, 91–99 (2012).
Kerschen, E.J. et al. Endotoxemia and sepsis mortality reduction by non-anticoagulant activated protein C. J. Exp. Med. 204, 2439–2448 (2007).
Ismail, J.A. et al. Immunohistologic labeling of murine endothelium. Cardiovasc. Pathol. 12, 82–90 (2003).
Conway, E.M., Nowakowski, B. & Steiner-Mosonyi, M. Human neutrophils synthesize thrombomodulin that does not promote thrombin-dependent protein C activation. Blood 80, 1254–1263 (1992).
Yerkovich, S.T. et al. Allergen-enhanced thrombomodulin (blood dendritic cell antigen 3, CD141) expression on dendritic cells is associated with a TH2-skewed immune response. J. Allergy Clin. Immunol. 123, 209–216 (2009).
Weiler-Guettler, H. et al. A targeted point mutation in thrombomodulin generates viable mice with a prethrombotic state. J. Clin. Invest. 101, 1983–1991 (1998).
Weiler, H. et al. Characterization of a mouse model for thrombomodulin deficiency. Arterioscler. Thromb. Vasc. Biol. 21, 1531–1537 (2001).
Weiler-Guettler, H., Aird, W.C., Husain, M., Rayburn, H. & Rosenberg, R.D. Targeting of transgene expression to the vascular endothelium of mice by homologous recombination at the thrombomodulin locus. Circ. Res. 78, 180–187 (1996).
Isermann, B. et al. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat. Med. 13, 1349–1358 (2007).
Burdelya, L.G. et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science 320, 226–230 (2008).
Johnson, S.M. et al. Mitigation of hematologic radiation toxicity in mice through pharmacological quiescence induced by CDK4/6 inhibition. J. Clin. Invest. 120, 2528–2536 (2010).
Weiler, H. Mouse models of thrombosis: thrombomodulin. Thromb. Haemost. 92, 467–477 (2004).
Connolly, A.J., Ishihara, H., Kahn, M.L., Farese, R.V. Jr. & Coughlin, S.R. Role of the thrombin receptor in development and evidence for a second receptor. Nature 381, 516–519 (1996).
Glaser, C.B. et al. Oxidation of a specific methionine in thrombomodulin by activated neutrophil products blocks cofactor activity. A potential rapid mechanism for modulation of coagulation. J. Clin. Invest. 90, 2565–2573 (1992).
Weisel, J.W., Nagaswami, C., Young, T.A. & Light, D.R. The shape of thrombomodulin and interactions with thrombin as determined by electron microscopy. J. Biol. Chem. 271, 31485–31490 (1996).
Kennel, S.J., Lankford, T., Hughes, B. & Hotchkiss, J.A. Quantitation of a murine lung endothelial cell protein, P112, with a double monoclonal antibody assay. Lab. Invest. 59, 692–701 (1988).
Modlich, U. et al. Cell-culture assays reveal the importance of retroviral vector design for insertional genotoxicity. Blood 108, 2545–2553 (2006).
Kustikova, O.S. et al. Cell-intrinsic and vector-related properties cooperate to determine the incidence and consequences of insertional mutagenesis. Mol. Ther. 17, 1537–1547 (2009).
Ross, C.C. et al. Inactivation of thrombomodulin by ionizing radiation in a cell-free system: possible implications for radiation responses in vascular endothelium. Radiat. Res. 169, 408–416 (2008).
Solis, M.M. et al. Recombinant soluble human thrombomodulin: a randomized, blinded assessment of prevention of venous thrombosis and effects on hemostatic parameters in a rat model. Thromb. Res. 73, 385–394 (1994).
Gupta, P.K. et al. Development of high-throughput HILIC-MS/MS methodology for plasma citrulline determination in multiple species. Anal. Meth. 3, 1759–1768 (2011).
Baddeley, A.J., Gundersen, H.J. & Cruz-Orive, L.M. Estimation of surface area from vertical sections. J. Microsc. 142, 259–276 (1986).
Langberg, C.W. & Hauer-Jensen, M. Optimal interfraction interval to minimize small bowel radiation injury in treatment regimens with two fractions per day: an experimental study in a rat model. Radiother. Oncol. 41, 249–255 (1996).
Kodell, R.L., Lensing, S.Y., Landes, R.D., Kumar, K.S. & Hauer-Jensen, M. Determination of sample sizes for demonstrating efficacy of radiation countermeasures. Biometrics 66, 239–248 (2010).
Acknowledgements
This work was supported by the US National Institutes of Health (grants CA71382 and AI67798 to M.H.-J., AI080557 to H.W., J.Á.F. and H.G., HL31950 and HL052246 to J.H.G., HL44612 to H.W. and CA122023 and AI080421 to D.Z.), the Edward P. Evans Foundation (M.H.-J., D.Z. and H.G.), the Ziegler Family Chair for Research (H.W.) and the Veterans Administration. We thank J. Bailey and V. Summey from the Comprehensive Mouse and Cancer Core at Cincinnati Children's Hospital Medical Center (CCHMC) for their support and services. We are grateful to M. Monestier (Temple University School of Medicine, Philadelphia, Pennsylvania, USA) for the BWA3 antibody, S.J. Kennel (University of Tennessee Graduate School of Medicine, Knoxville, Tennessee, USA) for the antibodies to Thbd (273-34A and 411-201B) and D. Gailani and A. Gruber (Vanderbilt University, Nashville, Tennessee, USA) for providing the 14E11 antibody.
Author information
Authors and Affiliations
Contributions
H.G. and H.W. designed and performed experiments, and wrote the paper. S.A.P. collected, analyzed and summarized data. E.J.K., I.H., H.P.H.L., J.A.C. and M.A.R. performed experiments. K.J.N. performed experiments and wrote parts of the paper. J.Á.F., A.S. and J.H.G. provided reagents and advised on experimental design. O.K., D.Z. and C.B. advised on experimental design and provided experimental expertise. Q.F. and J.W. performed in vivo studies. L.M.F. advised on experimental design and participated in writing the manuscript. K.-U.P. performed pharmacokinetics studies with Solulin and advised on experimental details. M.H.-J. designed experiments and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
K.-U.P. is employed by PAION Deutschland GmbH.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 and Supplementary Tables 1 and 2 (PDF 2140 kb)
Rights and permissions
About this article
Cite this article
Geiger, H., Pawar, S., Kerschen, E. et al. Pharmacological targeting of the thrombomodulin–activated protein C pathway mitigates radiation toxicity. Nat Med 18, 1123–1129 (2012). https://doi.org/10.1038/nm.2813
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2813
This article is cited by
-
Epidemiology of stroke and transient ischemic attacks in the population of the territories adjacent to the former Semipalatinsk Nuclear Test Site, Kazakhstan
Radiation and Environmental Biophysics (2022)
-
Fractionated radiation suppresses Kruppel-like factor 2 pathway to a greater extent than by single exposure to the same total dose
Scientific Reports (2020)
-
The fifth epidermal growth factor-like region of thrombomodulin exerts cytoprotective function and prevents SOS in a murine model
Bone Marrow Transplantation (2017)
-
Activated protein C protects from GvHD via PAR2/PAR3 signalling in regulatory T-cells
Nature Communications (2017)
-
3K3A–activated protein C stimulates postischemic neuronal repair by human neural stem cells in mice
Nature Medicine (2016)