Abstract
Transforming growth factor-β (TGF-β) has a pivotal function in the progression of renal fibrosis in a wide variety of renal diseases. Smad proteins have been identified to have an important function in regulating the expression of extracellular matrix (ECM) proteins through TGF-β signaling pathway. Aberrant TGF-β/Smad signaling can be modulated by stabilization of microtubules with paclitaxel. In this study, we investigated if paclitaxel can attenuate tubulointerstitial fibrosis in a rat model of unilateral ureteral obstruction (UUO). Rats in groups of six were subjected to UUO and received low-dose intraperitoneal injection of paclitaxel (0.3 mg/kg) twice a week. They were killed at day 7 and 14 after UUO or Sham operation. TGF-β signaling cascade and status of various ECM proteins were evaluated by RT–PCR, western blotting and immunohistochemical or immunofluorescence staining. The paclitaxel treatment markedly suppressed Smad2 and Smad3 phosphorylation. This was associated with attenuated expression of integrin-linked kinase, collagens I and III, fibronectin (FN) and α-smooth muscle actin, and a substantial decrease in renal fibrosis in animals that underwent UUO and received paclitaxel. These data indicate that the low-dose paclitaxel ameliorates renal tubulointerstitial fibrosis by modulating TGF-β signaling, and thus, the paclitaxel may have some therapeutic value in humans.
Similar content being viewed by others
Main
Renal interstitial fibrosis is an inevitable consequence of an increased activity of myofibroblasts and excessive accumulation of extracellular matrix (ECM) that occurs in virtually every type of chronic kidney disease. Renal interstitial fibrosis is a progressive process that ultimately leads to end-stage renal failure, which requires dialysis or kidney transplantation.1 Among various factors that regulate renal fibrosis, transforming growth factor-β (TGF-β) perhaps is the major cytokine that has a central function. TGF-β initiates the transition of renal tubular epithelial cells to myofibroblasts, the cellular source for ECM synthesis, which ultimately leads to irreversible renal failure.2, 3, 4 Blocking of TGF-β with the neutralizing antibodies, antisense oligonucleotides, decorin and siRNA strategies have provided strong evidence that targeting this molecule would be perhaps the best therapeutic maneuver that one can use to ameliorate renal fibrosis.5, 6, 7, 8 Other strategies may include the use of specific small molecules that could inhibit TGF-β receptor activity. However, transfer of such small molecules into humans still remains a daunting task, which is partly due to the inherent difficulties in methodologies associated with low efficiency and weak activity of nonviral vectors and unfavorable effects on the host body of viral vectors, such as, dysregulation of immune system and their oncogenic potential. Interestingly, a recent report shows that transgenic mice with TGF-β overexpression are actually protected from developing renal fibrosis, primarily through TGF-β's anti-inflammatory properties.9
The TGF-β evokes diverse cellular responses by binding to and activating specific cell-surface receptors that have intrinsic serine/threonine kinase activity. The activated TGF-β receptors stimulate the phosphorylation of receptor-regulated Smad2 and Smad3 proteins (R-Smads), which in turn form complexes with Smad4. This complex translocates from the cytoplasm into the nucleus, where the Smads regulate the transcription of target genes. Inhibitory Smad7 behaves in an opposing manner to the R-Smads, and downregulates TGF-β signaling.10 Some studies have previously shown that endogenous Smad2, Smad3 and Smad4 bind to microtubules in several cell lines, and the binding provides a negative regulatory mechanism to modulate TGF-β activity. Disruption of the microtubule network by chemical agents, such as, nocodazole and colchicine, leads to ligand-independent Smad nuclear accumulation and transcription of TGF-β-responsive genes, which in turn increases TGF-β-induced Smad activity.11 Also, there are certain literature reports that indicate aberrant ECM deposition in unilateral ureteral obstruction (UUO) model may be due to selective decrease of inhibitory Smad7 and increase of R-Smad3.12 The aim of this study was to assess if microtubule stabilization with low-dose paclitaxel (Taxol) could inhibit TGF-β/Smad signaling and ameliorate renal fibrosis in the UUO model.
MATERIALS AND METHODS
Care and Use of Laboratory Animals
Animal experiments were performed in accordance with the regulations set by the institutional committee for the care and use of laboratory animals, and were approved by the local authorities. Adult male Wistar rats (250–300 g body weight) were housed for 21–28 days on a 12 h light/dark cycle, and were allowed free access to food and water.
Animal Model
The animals were anesthetized with an intraperitoneal (IP) injection of freshly prepared Avertin. A midline incision was made in the abdominal wall, the left ureter was dissected out and ligated with 4.0 silk at two points along its length. The abdominal wound was approximated with the same silk suture and the animals returned to the cages.13 Four groups of rats comprising 24 animals each (total=96) were included as follows. (1) Sham group: on the day of operation these rats received IP injection of PBS twice a week. (2) Sham + Taxol group: the rats received IP injection of paclitaxel (Taxol; Sigma, St Louis, MO, USA) at a dose of 0.3 mg/kg twice a week. (3) UUO group: the rats received IP injection of PBS and underwent unilateral ureteral ligation. (4) UUO + Taxol group: these rats in addition those in group III received IP injection of Taxol. Half of the rats were killed on day 7 after UUO or Sham operation, and the other half on day 14. The kidneys were harvested for various biochemical and morphological studies.
Real-Time Polymerase Chain Reaction
Total RNA was isolated using the High Pure RNA Isolation Kit (Roche, Switzerland) according to the manufacturer's instructions. Contaminated DNA was removed by treating the samples with RNAase-free DNAase I (Promega, Madison, WI, USA). Real-time PCR was performed using Bio-Rad (Hercules, CA, USA) iQ SYBR Green supermix with Opticon (MJ Research, Waltham, MA, USA) following the vendor's instructions. Total RNA (100 μg) was reverse-transcribed and subjected to PCR as follows: 94°C for 2 min followed by 40 cycles of the following: 94°C for 15 s, 58°C for 30 s and 72°C for 30 s, and a final extension at 72°C for 10 min. The primers used were as follows. Rat Smad2, forward: 5′-TCACAGCCATCATGAGCTCAAGG-3′, reverse: 5′-TGTGACGCATGGAAGGTCTCTC-3′; Smad3, forward: 5′-AGCACACAATAACTTGG ACC-3′, reverse: 5′-TAAGACACACTGGAACAGCGGATG-3′; integrin-linked kinase (ILK), forward: 5′-CCGCTGGCAGGGCAATGACATT-3′, reverse: 5′-GGGGGAGCCTGGCAAGCACCTA-3′; α-smooth muscle actin (α-SMA), forward: 5′-ACTGGGACGACATGGAAAAG-3′, reverse: 5′-CATCTCCAGAGTCCAGCACA-3′; collagen I, forward: 5′-GAGCGGAGAGTACTGGATCG-3′, reverse: 5′-TACTCGAACGGGAATCCATC-3′; collagen III, forward: 5′-TGGTCCTCAGGGTGTAAAGG-3′, reverse: 5′-GTCCAGCATCACCTTTTGGT-3′; fibronectin (FN), forward: 5′-TGACTCGCTTTGACTTCACCAC-3′, reverse: 5′-TCTCCTTCCTCGCTCAGTTCGT-3′. All samples were subjected to reverse transcription (RT)–PCR along with the housekeeping gene GAPDH having the following primer sequences: forward, 5′-TGCTGAGTATGTCGTGGAGTCTA-3′; reverse, 5′-AGTGGGAGTTGCTGTTGAAATC-3′ as an internal standard. Reaction specificity was confirmed by gel electrophoresis of products after real-time PCR and melting curve analysis. Ratios for Smad2/GAPDH, Smad3/GAPDH, α-SMA/GAPDH and collagen I/GAPDH, collagen III/GAPDH, FN/GAPDH mRNA were calculated for each sample and expressed as the means±s.d.
Histology and Immunohistochemistry
For histological analysis, kidney tissues fixed with 4% buffered paraformaldehyde were embedded in paraffin, and 3-μm-thick sections were prepared. The sections were then stained with PAS and Masson's trichrome.13 Immunohistochemical analyses were carried out by using anti-phospho-Smad2 (Upstate Biotechnology), anti-phospho-Smad3 (Cell Signaling Technology), anti-collagen III (Abcam), anti-collagen-I and anti-α-SMA (Santa Cruz Biotechnology). The sections were deparaffinized and quenched in 3% H2O2 for 10 min to block endogenous peroxidase and then washed in PBS. They were incubated with various primary antibodies as listed above for 1 h and then with biotinylated secondary antibody followed by ABC reagent treatment as recommended by the vendor. Color development was achieved by incubating the sections with diaminobenzidine as a substrate. Slides were counterstained with Mayer's hematoxylin. Preincubation of the primary antibody with specific blocking peptides or substitution of the primary antibody with an irrelevant IgG served as negative controls.4 For immunofluorescence study of FN, cryostat sections (4-μm thick) fixed in cold acetone were incubated with mouse anti-FN (Santa Cruz Biotechnology) for 1 h, followed by incubation with FITC-labeled anti-mouse IgG (Biosource, Camarillo, CA, USA). The slides were developed by confocal laser scanning microscopy. The intensity of phospho-Smad2, phospho-Smad3, α-SMA, collagen I, collagen III and FN staining was analyzed by an image analysis software (Path QC; Logene Biological Medical Engineering).
Western Blotting
Western blot analyses were carried out for the detection of Smad2/3, phosphorylated Smad2/3, α-SMA, collagen I, collagen III and FN expression in the kidneys as described.14 Briefly, kidney tissues were treated with a lysis buffer (Sigma) and a cocktail set of phosphatase inhibitors (Calbiochem). Samples (20 μg) were fractionated by sodium dodecylsulfate–polyacrylamide gel electrophoresis. After transfer onto nitrocellulose membrane (Amersham, Buckinghamshire, UK), the blots were probed with a mouse monoclonal antibody to α-SMA (Santa Cruz Biotechnology; 1:1000 dilution) or a rabbit polyclonal antibody to p-Smad3 (Cell Signaling Technology; 1:1000 dilution) or ILK (Santa Cruz Biotechnology; 1:1000 dilution) or a goat polyclonal antibody p-Smad2 (Upstate Biotechnology; 1:1000 dilution) and collagen I (Santa Cruz Biotechnology; 1:2000 dilution), collagen III (Abcam; 1:2000 dilution), FN (Santa Cruz Biotechnology; 1:1000 dilution) and peroxidase-conjugated goat anti-mouse IgG (1:20 000 dilution). The swine anti-rabbit IgG or rabbit anti-goat in PBS containing 1% normal goat serum and 1% FCS and β-actin were used as internal controls.
Statistical Analysis
Data were calculated as the mean±s.d. and the groups were compared using one-way analysis of variance. Statistical significance was set at P<0.05.
RESULTS
Effects of Paclitaxel on Renal Histology in Rat UUO Model
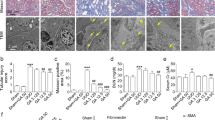
The paraffin-embedded sections of UUO or animals treated with paclitaxel were stained with PAS and examined. The UUO animals exhibited significant tubular atrophy on days 7 and 14, which was notably reduced with the paclitaxel treatment (Figure 1).
PAS-stained paraffin-embedded rat kidney sections (a) from Sham group, (b) 7 days after UUO, (c) 7 days after UUO + Taxol, (d) 14 days after UUO and (e) 14 days after UUO + Taxol. The UUO groups show severe tubular atrophy, which was apparently ameliorated by paclitaxel treatment. Original magnifications, × 200.
Inhibition of ECM Expression is a Central Mechanism by which Paclitaxel Prevents Progressive Renal Injury in Rat Obstructive Nephropathy
The suppressive effects of paclitaxel on tubulointerstitial fibrosis, and mRNA and protein expression of ILK, α-SMA, collagen I, collagen III and FN were assessed by real-time RT-PCR, western blotting and immunohistochemistry. Figure 2 shows Masson's trichrome staining of tissue sections from UUO or animals treated with paclitaxel. The UUO animals exhibited marked tubulointerstitial fibrosis on days 7 and 14, which was significantly reduced by paclitaxel treatment. Figure 3 shows RT–PCR analysis that indicates paclitaxel has no effect on the basal mRNA expression of ILK, α-SMA, collagen I, collagen III and FN in Sham-operated rats. The expression of ILK, α-SMA, collagen I, collagen III and FN was reduced with paclitaxel treatment in kidney of rats with UUO on days 7 and 14 (P<0.01, n=6). Western blot and immunohistochemistry or immunofluorescence analyses reveal similar trends as of mRNA expression (P<0.05, n=6; Figures 4, 5, 6, 7 and 8).
Treatment with Taxol suppresses interstitial fibrosis. (A) Representative Masson's trichrome-stained sections. (a) Sham group, (b) 7 days after UUO, (c) 7 days after UUO + Taxol, (d) 14 days after and (e) 14 days after UUO + Taxol. (B) Semiquantitative score of tubulointerstitial fibrosis in the cortex of the kidneys. Each bar represents the mean±s.d. for six animals. ΔP<0.01 vs sham group (n=6); #P<0.05 vs UUO group. Original magnifications, × 400.
Real-time PCR depicting ILK, α-SMA, collagen I, FN and collagen III mRNA expression in vivo after paclitaxel treatment in UUO model. (a) ILK mRNA, (b) α-SMA mRNA, (c) collagen I mRNA, (d) FN mRNA and (e) collagen III mRNA. ILK, α-SMA, collagen I, FN and collagen III expression were significantly higher in UUO rats on days 7 and 14 compared to Sham animals or Sham animals treated with paclitaxel (P<0.05, n=6). Treatment with paclitaxel resulted in a decrease in ILK, α-SMA, collagen I, FN and collagen III expression (P<0.05, n=6). Densitometric analyses were performed from six independent experiments. Each bar represents the mean±s.d. for six animals. ΔP<0.01 vs sham group or Sham + Taxol group (n=6); #P<0.05 vs UUO group.
Western blot analyses depicting ILK, α-SMA, collagen I, FN and collagen III expression in vivo after paclitaxel treatment in UUO model. Whole renal cell extracts were immunoblotted with the indicated antibodies. ILK, α-SMA, collagen I, FN and collagen III expression was significantly higher in UUO rats on days 7 and 14 (P<0.01, n=6) than in Sham animals or Sham animals treated with paclitaxel. Treatment with paclitaxel resulted in a decrease in ILK, α-SMA, collagen I, FN and collagen III expression (P<0.05, n=6). Densitometric analyses were performed from six independent experiments. Each bar represents the mean±s.d. for six animals. ΔP<0.01 vs Sham group or Sham + Taxol group (n=6); #P<0.05 vs UUO group.
Taxol treatment suppresses collagen I expression in UUO model. (A) Representative rat kidney sections subjected to collagen I immunohistochemistry. (a) Sham group, (b) 7 days after UUO, (c) 7 days after UUO + Taxol, (d) 14 days after UUO and (e) 14 days after UUO + Taxol. Renal collagen I expression was markedly higher in UUO rats on days 7 and 14 compared to Sham animals (P<0.01, n=6). Treatment with paclitaxel resulted in a decrease in collagen I expression (P<0.05, n=6). (B) Densitometric analyses were performed from six independent experiments. Each bar represents mean±s.d. for six animals. ΔP<0.01 vs Sham group (n=6); # P<0.05 vs UUO group. Original magnifications, × 400.
Taxol treatment suppresses α-SMA expression in UUO model. (A) Representative rat kidney sections subjected to α-SMA immunohistochemistry. (a) Sham group, (b) 7 days after UUO, (c) 7 days after UUO + Taxol, (d) 14 days after UUO and (e) 14 days after UUO + Taxol. Renal α-SMA expression was markedly higher in UUO rats on days 7 and 14 compared to Sham animals (P<0.01, n=6). Treatment with paclitaxel resulted in a decrease in α-SMA expression (P<0.05, n=6). (B) Densitometric analyses were performed from six independent experiments. Each bar represents mean±s.d. for six animals. ΔP<0.01 vs Sham group (n=6); #P<0.05 vs UUO group. Original magnifications, × 400.
Taxol treatment suppresses FN expression in UUO model. (A) Representative rat kidney sections subjected to FN immunofluorescence. (a) Sham group, (b) 7 days after UUO, (c) 7 days after UUO + Taxol, (d) 14 days after UUO and (e) 14 days after UUO + Taxol. Renal FN expression was markedly higher in UUO rats on days 7 and 14 compared to Sham animals (P<0.01, n=6). Treatment with paclitaxel resulted in a decrease in FN expression (P<0.05, n=6). (B) Densitometric analyses were performed from six independent experiments. Each bar represents mean±s.d. for six animals. ΔP<0.01 vs Sham group (n=6); #P<0.05 vs UUO group. Original magnifications, × 200.
Taxol treatment suppresses collagen III expression in UUO model. (A) Representative rat kidney sections subjected to collagen III immunohistochemistry. (a) Sham group, (b) 7 days after UUO, (c) 7 days after UUO + Taxol, (d) 14 days after UUO and (e) 14 days after UUO + Taxol. Renal collagen I expression was markedly higher in UUO rats on days 7 and 14 compared to Sham animals (P<0.01, n=6). Treatment with paclitaxel resulted in a decrease in collagen III expression (P<0.05, n=6). (B) Densitometric analyses were performed from six independent experiments. Each bar represents mean±s.d. for six animals. ΔP<0.01 vs Sham group (n=6); #P<0.05 vs UUO group. Original magnifications, × 200.
Blockade of Smad2/3 Activation is a Key Mechanism by which Paclitaxel Prevents Renal Fibrosis in Obstructive Nephropathy
The effects of paclitaxel on expression of Smad2 and Smad3 mRNA by real-time RT-PCR were assessed. The analysis shows that paclitaxel has no effect on Smad2 and Smad3 mRNA expression in Sham-operated or UUO rat kidneys on days 7 and 14 (Figure 9). Western blot analysis shows that phosphorylated Smad2/3 but not ‘total’ Smad2 or Smad3 expression were markedly reduced with paclitaxel (Taxol) treatment in kidneys of rats with UUO on days 7 and 14 (P<0.01, n=6). By contrast, paclitaxel has no effect on the expression and phosphorylation of Smad2/3 in Sham-operated rat kidneys (Figure 10). Immunohistochemistry analysis indicated similar results (Figures 11 and 12).
Real-time PCR showing Smad2 (a) and Smad3 (b) mRNA expression in vivo after treatment with paclitaxel in UUO model. Smad2 and Smad3 mRNA expression was more or less similar in Sham and UUO animals treatment with paclitaxel on days 7 and 14. Densitometric analyses were performed from six independent experiments. Each bar represents the mean±s.d. for six animals. ΔP<0.01 vs Sham group or Sham + Taxol group (n=6); #P<0.05 vs UUO group.
Western blot analysis showing Smad2 and Smad3 protein expression in vivo after treatment with paclitaxel in UUO model. Phosphorylated Smad2 and Smad3 but not total Smad2 or Smad3 expression was significantly higher in UUO rats on days 7 and 14 (P<0.01, n=6) compared with Sham or Sham animals treated with paclitaxel. Treatment with paclitaxel resulted in a decrease in phosphorylation of Smad2 and Smad3 (P<0.05, n=6). Densitometric analyses were performed from six independent experiments. Each bar represents the mean±s.d. for six animals. ΔP<0.01 vs Sham group or Sham + Taxol group (n=6); #P<0.05 vs UUO group.
Treatment with Taxol suppresses phosphorylated Smad2 expression in UUO model. (A) Representative rat kidney sections were treated with anti-phosphorylated Smad2 antibody. (a) Sham group, (b) 7 days after UUO, (c) 7 days after UUO + Taxol, (d) 14 days after UUO and (e) 14 days after UUO + Taxol. Renal phosphorylated Smad2 expression was markedly higher in UUO rats on days 7 and 14 compared with Sham animals (P<0.01, n=6). Treatment with paclitaxel resulted in a decrease in phosphorylated Smad2 expression (P<0.05, n=6). (B) Densitometric analyses were performed from six independent experiments. Each bar represents mean±s.d. for six animals. ΔP<0.01 vs Sham group (n=6); #P<0.05 vs UUO group. Original magnifications, × 400.
Treatment with Taxol suppresses phosphorylated Smad3 expression in UUO model. (A) Representative rat kidney sections were treated with anti-phosphorylated Smad3 antibody. (a) Sham group, (b) 7 days after UUO, (c) 7 days after UUO + Taxol, (d) 14 days after UUO and (e) 14 days after UUO + Taxol. Renal phosphorylated Smad3 expression was markedly higher in UUO rats on days 7 and 14 compared with Sham animals (P<0.01, n=6). Treatment with paclitaxel resulted in a decrease in phosphorylated Smad3 expression (P<0.05, n=6). (B) Densitometric analyses were performed from six independent experiments. Each bar represents mean±s.d. for six animals. ΔP<0.01 vs Sham group (n=6); #P<0.05 vs UUO group. Original magnifications, × 400.
DISCUSSION
In this study, we observed that the renal tubulointerstitial fibrosis in the UUO model on days 7 and 14 was substantially decreased by IP injection of paclitaxel, a microtubule-stabilizing agent, thus suggesting that the low-dose paclitaxel may have therapeutic benefits in the amelioration of renal tubulointerstitial fibrosis.
Renal fibrosis is an end point of a wide variety of renal diseases that leads to an end-stage renal disease, and TGF-β has been regarded as a key mediator in the progression of the process of fibrosis. TGF-β is upregulated in UUO model, which is associated with tubular epithelial–mesenchymal transition (EMT) and increased synthesis of ECM.15 The downstream signaling effects of TGF-β are mediated by Smad3.16 The Smad3 loss can afford protection from radiation-induced fibrosis,17 bleomycin-induced pulmonary fibrosis,18 presumably by interrupting the pathways leading up to matrix production by fibroblasts that lead to tubulointerstitial fibrosis. Recent reports indicate that Smad7 is selectively decreased, whereas phosphorylation of Smad2 and Smad3 is increased in UUO model.12 Furthermore, it has been previously shown in several different cell lines that microtubules serve as negative regulator for TGF-β/Smad signaling by forming a complex with endogenous Smad2, Smad3 and Smad4, thus sequestering the R-Smads away from the TGF-β receptor.11 Therefore, it is conceivable that stabilization of microtubules by low-dose paclitaxel can dampen the exacerbated TGF-β signaling as reported in TGF-β-induced inhibition of myogenesis in C2C12 myoblasts.19 Similarly, in an earlier report, Liu et al20 also described that paclitaxel can significantly suppress TGF-β/Smad activity in SCID mice. In this study, we provide evidence that low-dose paclitaxel suppresses phosphorylation of Smad2 and Smad3, two homologous Smad proteins that transduce signals from TGF-β and activin, in rat UUO model. These data support the notion that TGF-β/Smad signaling is regulated by the dynamic stability/instability of microtubules that are sensitive to low-dose microtubule-stabilizing agents, such as paclitaxel.
Paclitaxel is an anticancer agent,21 which by stabilizing polymerized microtubules and enhancing microtubule assembly, arrests the cell cycle in the G0/G1 and G2/M phases, leading to cell death.22, 23 Prolonged chemotherapeutic treatment with paclitaxel has been associated with scleroderma-like changes or pulmonary fibrosis, albeit in only a small fraction of the patients. It is noteworthy that the inhibition of tumor cell proliferation can be achieved by much higher dosages of paclitaxel. The inhibition of TGF-β/Smad signaling, however, can be attained with very low dose of paclitaxel. Whereas, some of the recent studies indicate that low-dose paclitaxel had minimal, if any, detectable effects on cell proliferation and other cellular activities, including fibrosis. Intriguingly, low-dose paclitaxel has been shown to inhibit collagen-induced arthritis and fibrosis associated with systemic sclerosis in SCID mice.20, 24, 25 In this study, the low-dose paclitaxel treatment effectively reduced interstitial deposition of collagen I, collagen III and FN, and expression of ILK and α-SMA. The ILK is an intracellular serine/threonine protein kinase that interacts with the cytoplasmic domains of β-integrins and several other cytoskeleton-associated proteins.26, 27, 28, 29 Increasing evidence suggest that ILK is a critical mediator for tubular EMT and most likely has a crucial function in the pathogenesis of renal fibrosis, where ILK induction by TGF-β1 is clearly dependent on Smad signaling in tubular epithelial cells.30 The expression of α-SMA is considered specific for myofibroblasts that may be derived from EMT. The myofibroblasts expressing α-SMA are found in the interstitium of obstructed kidneys and such cells may produce ECM components, ie, collagen I, collagen III and FN,31 similar to the ones observed in the rat UUO model.
In conclusion, we have shown that low-dose paclitaxel can significantly suppress the exacerbated TGF-β/Smad/ILK signaling in kidney and lessens the interstitial fibrosis in the rat UUO model by reduction of TGF-β expression. It is hoped that the results of current studies would give an impetus for future investigations to explore the therapeutic potential of paclitaxel in the amelioration of renal tubulointerstitial fibrosis.
References
Schieppati A, Remuzzi G . Chronic renal diseases as a public health problem: epidemiology, social, and economic implications. Kidney Int 2005;98:S7–S10.
Zeisberg M, Maeshima Y, Mosterman B, et al. Renal fibrosis. Extracellular matrix microenvironment regulates migratory behavior of activated tubular epithelial cells. Am J Pathol 2002;160:2001–2008.
Lan HY . Tubular epithelial-myofibroblast transdifferentiation mechanisms in proximal tubule cells. Curr Opin Nephrol Hypertens 2003;12:25–29.
Li JH, Zhu HJ, Huang XR, et al. Smad7 inhibits fibrotic effect of TGF-beta on renal tubular epithelial cells by blocking Smad2 activation. J Am Soc Nephrol 2002;13:1464–1472.
Imai E, Isaka Y . Strategies of gene transfer to the kidney. Kidney Int 1998;53:264–272.
Fine LG . Gene transfer into the kidney: promise for unraveling disease mechanisms, limitations for human gene therapy. Kidney Int 1996;49:612–619.
Akagi Y, Isaka Y, Arai M, et al. Inhibition of TGF-beta 1 expression by antisense oligonucleotides suppressed extracellular matrix accumulation in experimental glomerulonephritis. Kidney Int 1996;50:148–155.
Isaka Y, Brees DK, Ikegaya K, et al. Gene therapy by skeletal muscle expression of decorin prevents fibrotic disease in rat kidney. Nat Med 1996;2:418–423.
Wang W, Huang XR, Li AG, et al. Signaling mechanism of TGF-beta1 in prevention of renal inflammation: role of Smad7. J Am Soc Nephrol 2005;16:1371–1383.
Derynck R, Zhang YE . Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003;425:577–584.
Dong C, Li Z, Alvarez R, et al. Microtubule binding to Smads may regulate TGF beta activity. Mol Cell 2000;5:27–34.
Fukasawa H, Yamamoto T, Togawa A, et al. Down-regulation of Smad7 expression by ubiquitin-dependent degradation contributes to renal fibrosis in obstructive nephropathy in mice. Proc Natl Acad Sci USA 2004;101:8687–8692.
Hu B, Wu Z, Phan SH . Smad3 mediates transforming growth factor-beta-induced alpha-smooth muscle actin expression. Am J Respir Cell Mol Biol 2003;29:397–404.
Dooley S, Delvoux B, Streckert M, et al. Transforming growth factor beta signal transduction in hepatic stellate cells via Smad2/3 phosphorylation, a pathway that is abrogated during in vitro progression to myofibroblasts. TGF-beta signal transduction during transdifferentiation of hepatic stellate cells. FEBS Lett 2001;502:4–10.
Sato M, Muragaki Y, Saika S, et al. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest 2003;112:1486–1494.
Flanders KC . Smad3 as a mediator of the fibrotic response. Int J Exp Pathol 2004;85:47–64.
Flanders KC, Sullivan CD, Fujii M, et al. Mice lacking Smad3 are protected against cutaneous injury induced by ionizing radiation. Am J Pathol 2002;160:1057–1068.
Zhao J, Shi W, Wang YL, et al. Smad3 deficiency attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol 2002;282:585–593.
Zhu S, Goldschmidt-Clermont PJ, Dong C . Transforming growth factor-beta-induced inhibition of myogenesis is mediated through Smad pathway and is modulated by microtubule dynamic stability. Circ Res 2004;94:617–625.
Liu X, Zhu S, Wang T, et al. Paclitaxel modulates TGFbeta signaling in scleroderma skin grafts in immunodeficient mice. PLoS Med 2005;2:1334–1442.
Gelmon K . The taxoids: paclitaxel and docetaxel. Lancet 1994;344:1267–1272.
Donaldson KL, Goolsby GL, Kiener PA, et al. Activation of p34cdc2 coincident with taxol-induced apoptosis. Cell Growth Differ 1994;5:1041.
Schiff PB, Fant J, Horwitz SB . Promotion of microtubule assembly in vitro by taxol. Nature 1979;277:665–667.
Brahn E, Tang C, Banquerigo ML . Regression of collagen-induced arthritis with taxol, a microtubule stabilizer. Arthritis Rheum 1994;37:839–845.
Cao L, Sun D, Cruz T, et al. Inhibition of experimental allergic encephalomyelitis in the Lewis rat by paclitaxel. J Neuroimmunol 2000;108:103–111.
Wu C . ILK interactions. J Cell Sci 2001;114:2549–2550.
Wu C, Dedhar S . Integrin-linked kinase (ILK) and its interactors: a new paradigm for the coupling of extracellular matrix to actin cytoskeleton and signaling complexes. J Cell Biol 2001;155:505–510.
Dedhar S, Williams B, Hannigan G . Integrin-linked kinase (ILK): a regulator of integrin and growth-factor signalling. Trends Cell Biol 1999;9:319–323.
Dedhar S . Cell-substrate interactions and signaling through ILK. Curr Opin Cell Biol 2000;12:250–256.
Li Y, Yang J, Dai C, et al. Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. J Clin Invest 2003;112:503–516.
Liu Y . Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol 2004;15:1–12.
Acknowledgements
This work was supported by grants from the Creative Research Group Fund of the National Foundation Committee of Natural Science of China (30871169/C140405), and grants from the NIH (DK28492 and DK60635).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Disclosure/Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, D., Sun, L., xian, W. et al. Low-dose paclitaxel ameliorates renal fibrosis in rat UUO model by inhibition of TGF-β/Smad activity. Lab Invest 90, 436–447 (2010). https://doi.org/10.1038/labinvest.2009.149
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2009.149
Keywords
This article is cited by
-
Methyl-CpG-binding domain protein 2 contributes to renal fibrosis through promoting polarized M1 macrophages
Cell Death & Disease (2022)
-
DsbA-L mediated renal tubulointerstitial fibrosis in UUO mice
Nature Communications (2020)
-
Paclitaxel-coated stents to prevent hyperplastic proliferation of ureteral tissue: from in vitro to in vivo
Urolithiasis (2020)
-
p53 induces miR199a-3p to suppress SOCS7 for STAT3 activation and renal fibrosis in UUO
Scientific Reports (2017)
-
MBD2 upregulates miR-301a-5p to induce kidney cell apoptosis during vancomycin-induced AKI
Cell Death & Disease (2017)