Abstract
Administration of a single low dose of the N-methyl-D-aspartate (NMDA) receptor antagonist ketamine has been demonstrated to elicit long-lasting antidepressant effects in humans with depression, as well as in rodent models of depression. Although pharmacological studies have implicated the GluN2B subunit of the NMDA receptor in these effects, drugs targeting this subunit have off-target actions, and systemic administration of these compounds does not allow for delineation of specific brain regions involved. In this study, we assessed the role of GluN2B in the bed nucleus of the stria terminalis (BNST) in novelty-induced hypophagia (NIH) in mice. First, we verified that ketamine, as well as the GluN2B antagonist Ro25–6981, decreased the latency to consume food in a novel environment in a version of the NIH test. We then hypothesized that GluN2B-containing receptors within the BNST may be a target of systemic ketamine and contribute to behavioral effects. Through the combination of a GluN2B-floxed mouse line and stereotaxic delivery of lentiviral Cre recombinase, we found that targeted knockdown of this subunit within the BNST mimicked the reduction in affective behavior observed with systemic ketamine or Ro25–6981 in the NIH test. These data suggest a role for GluN2B-containing NMDARs within the BNST in the affective effects of systemic ketamine.
Similar content being viewed by others
Introduction
A single, subanesthetic dose of the N-methyl D-aspartate receptor (NMDAR) antagonist ketamine exerts a rapid antidepressant action in patients with depression,1 and has been associated with a number of adaptations throughout the brain. However, ketamine acts as a psychotomimetic and has abuse potential, so understanding which specific adaptations are contributing to its positive behavioral effects is crucial for safer treatment options for depression. In this study, we propose that GluN2B subunit-containing NMDARs within the bed nucleus of the stria terminalis (BNST) contribute to the actions of ketamine in a novelty-induced hypophagia (NIH) task used to test antidepressant efficacy.
Preclinical rodent studies using ketamine have mirrored the antidepressant actions observed in humans,2,3 with studies using the GluN2B-selective antagonist Ro25–6981 specifically implicating GluN2B-containing NMDARs. In rodents, systemic ketamine administration enhances glutamate synaptic function in the medial prefrontal cortex and hippocampus through regulation of homeostatic plasticity.3 Currently however, specific populations of NMDARs have not been linked to antidepressant behavioral effects. We pursued the anatomical underpinning of ketamine’s antidepressant actions, focusing on the BNST. The BNST is a component of the extended amygdala that modulates affective behavior, has been implicated in depression and anxiety, and has relatively high adult expression of GluN2B-containing NMDARs.4, 5, 6 Further, activation of glutamate synapses in the BNST drives negative affect-related behaviors.7 Given the evidence for GluN2B-containing NMDARs in the antidepressant effects of ketamine, we hypothesized that these receptors within the BNST might govern affective behaviors.
Materials and methods
Animals and treatment
All animals were housed 2–5 per cage and were provided with rodent chow and tap water ad libitum. The temperature- and humidity-controlled animal facilities are maintained on a 12:12 -h light:dark cycle (lights on 0600–1800 hours). All experiments took place during the light phase of the cycle.
For ketamine and Ro25–6981 experiments, male wild-type C57Bl/6J mice (Jackson Laboratory, Bar Harbor, ME, USA; 8 weeks) were acclimated to the vivarium for a minimum of 1 week prior to experimentation. Following acclimation, mice were handled for 5 days and given habituating saline injections for the last 3 days of handling. Ketamine (3 mg kg−1 body weight, Ketaved; Webster Veterinary Supply, Sterling, MA, USA), Ro 25–6981 maleate (5 mg kg−1; Tocris, Minneapolis, MN, USA) and 0.9% saline were administered intraperitoneally (i.p.).
Floxed Grin2b mice were generated by Eric Delpire as previously described,8 and floxed Nr3c1 (GR) mice were generated by Louis Muglia as previously described.9 Both lines were bred homozygous for the floxed allele in our facility and underwent surgery (described below) at 1–4 months of age. Prior to behavioral testing, mice were handled for 5 days.
All protocols were approved by the Vanderbilt University Institutional Animal Care and Use Committee.
Surgical procedure
Mice were anesthetized with 1.5% isoflurane. Then, 200 nl LV-CRE (UNC Vector Core; Titer=1.3 × 109) or LV-GFP (UNC Vector Core; Titer=1.3 × 1010) was injected into the dorsal BNST (coordinates: AP: 0.14 mm; L: ±0.88 mm; D: −4.24 mm) at a rate of 50 nl/min using an AngleTwo stereotax at a 15.03° angle to avoid ventricles. Postoperatively, mice were administered ketoprofen (5 mg kg−1 subcutaneously) once per day for 72 h, then p.r.n. for 1 week. Mice were given at least 2 weeks recovery time following surgery before behavioral experimentation.
Novelty-induced hypophagia (NIH)
The NIH test consisted of 4 training days followed by a testing day. On all days, mice were given at least 1 h acclimation to the testing room under low red light (~40 lux), and all mice had access to rodent chow throughout behavioral testing. During training, mice were given 30 min per day access to a highly palatable food (liquid Ensure, home-made vanilla shake flavor) in the testing room while group-housed in their home cages under low red light. By the second training day, all mice had consumed Ensure, so no mice were excluded from the study. On the test day in the ketamine and Ro25–6981 study, half of the mice were given a 1 -h restraint stress in 50- ml conical tubes, while the other half were allowed to remain in their home cages. Half an hour following the termination of restraint stress, all mice were given i.p. injections of ketamine, Ro25–6981 or equal-volume saline, yielding six groups (no restraint-saline, no restraint-ketamine, no restraint-Ro, restraint-saline, restraint-ketamine and restraint-Ro). On the testing day, each mouse was transferred to an individual novel cage devoid of bedding under bright lighting (~200 lux) immediately prior to 30 min Ensure access. Cages were cleaned with 30% EtOH before and after each animal.
Data and statistical analysis
Latency (s) to the first sip of Ensure and amount (g) consumed were measured in the NIH test. For ketamine and Ro25–6981 studies, statistical significance was calculated via two-way analysis of variance (ANOVA) for treatment x restraint with a post-hoc Bonferroni multiple comparison test in the NIH test. For all studies using transgenic animals, statistical significance was calculated via t-test. All data were analyzed using GraphPad Prism 5 (La Jolla, CA, USA). Data are represented as mean±s.e.m. Significance was set at P<0.05.
Results and Discussion
We first validated a version of the NIH test for analysis of the antidepressant efficacy of NMDAR manipulation. In brief, the latency to consume food in a novel environment is quantified as a measure of the affective state. Previous studies used chronically stressed rodents and/or food restriction in similar paradigms.2,3 We used a protocol similar to that described by Dulawa and Hen, in which satiated mice seek a highly palatable food reward.10 This test, thus, is thought to rely on hedonic drive to consume rather than hunger, which may be more closely aligned with depression-associated anhedonia.
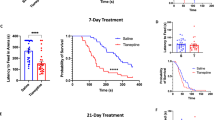
Mice received ketamine (3 mg kg−1, i.p.), Ro25–6981 (5 mg kg−1, i.p.) or equivalent volume saline for half an hour following a 1-h restraint stressor or no stress. The amount of Ensure consumed and the latency to consume the first sip of Ensure were measured, with increased latency indicating increased negative affective behavior. We found that ketamine and Ro25–6981 each significantly reduced this latency (F(2, 72)=12.46, P<0.0001) in mice, regardless of whether the mice had been previously treated with a 1-h restraint stressor or not (Figure 1a). No difference in consumption was observed with ketamine or Ro25–6981 administration (Supplementary Figure 1). We examined the effect of ketamine and Ro25–6981 administration on anxiety-like behavior in the elevated zero maze (EZM) following a forced swim stressor (Supplementary Methods), and observed no difference in open arm time or distance traveled (Supplementary Figure 1).
Viral deletion of GluN2B from the bed nucleus of the stria terminalis (BNST) phenocopies systemic treatment with ketamine or Ro25–6981. (a) Timeline above. Decreased latencies with systemic ketamine (3 mg kg−1) and Ro25 (5 mg kg−1) administration in our novelty-induced feeding suppression paradigm were repeated with (R) and without (NR) restraint stress. (b) Coronal atlas image (left) showing dorsal BNST in the inset and LV-GFP injection targeting (right). (c) Viral-mediated deletion of GluN2B from BNST (LV-Cre) phenocopies systemic ketamine; (d) however, viral-mediated deletion of GR from the BNST has no such effect. Data are presented as means with s.e.m. *P>0.05; **P>0.01. Ns are indicated on bars.
Although Ro25–6981 is selective for GluN2B-containing NMDARs over non-GluN2B-containing NMDARs, this compound acts on a number of off-target sites also implicated in depression, including serotonin and norepinephrine transporters.11 Thus, we sought to determine specific neural circuits where GluN2B-NMDARs influence antidepressant action by utilizing a mouse line harboring floxed Grin2b alleles5 in concert with stereotaxic delivery of lentiviral Cre recombinase (LV-Cre) to knock down GluN2B expression within the BNST. This GluN2B-floxed line has been used in previous studies by our lab and, when crossed with mice expressing tetO-Cre under the CaMKII promoter, demonstrated 80% reduction in GluN2B levels in the BNST as assessed by western blot.5 Lentiviral GFP (LV-GFP) was injected as a control as previously described (Figure 1b),12 and the functionality of Cre recombinase was confirmed through LV-Cre injection into the BNST of the Ai9tomato reporter mouse line (data not shown). LV-Cre-injected animals displayed a significant decrease in NIH latency compared to LV-GFP controls (t(45)=2.44, P=0.0186; Figure 1c), mirroring ketamine- or Ro25–6981-treated animals. No difference in total consumption in the NIH test or anxiety-like behavior as measured by the EZM was observed (Supplementary Figure 1). It is interesting to note that when GluN2B knockdown in this floxed line is limited to corticohippocampal regions, with no deficit in amygdalar GluN2B expression, no impairment in affective behavior is observed.8 This indicates that the behavioral phenotype observed in the study outlined here is regionally specific for GluN2B within the BNST.
To control for potential nonspecific actions of the LV-Cre, we performed a parallel study using LV-Cre and LV-GFP injection in a previously described floxed glucocorticoid receptor (GRfl/fl) line. In these studies, LV-Cre-injected GRfl/fl mice performed similarly to LV-GFP-injected mice (Figure 1d). These data indicate that knockdown of GluN2B from the BNST, not nonspecific actions of LV-Cre administration, reduced negative affective behavior.
Taken together, these data indicate that GluN2B-containing NMDARs within the BNST play an important role in regulating depression. Further, our pharmacological data demonstrate that ketamine and Ro25–6981 exert antidepressant-like effects in the NIH paradigm without need of prior stress exposure. Future studies will aim to elucidate the mechanism by which GluN2B blockade in the BNST exerts antidepressant-like effects, and determine if direct pharmacological inhibition of GluN2B within the BNST through cannulated injection of ketamine or Ro25–6981 is able to recapitulate the behavioral effects observed with systemic administration.
References
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS et al Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 2000; 47: 351–354.
Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H et al Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 2011; 69: 754–761.
Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF et al NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 2011; 475: 91–95.
Sink KS, Walker DL, Freeman SM, Flandreau EI, Ressler KJ, Davis M . Effects of continuously enhanced corticotropin releasing factor expression within the bed nucleus of the stria terminalis on conditioned and unconditioned anxiety. Mol Psychiatry 2013; 18: 308–319.
Wills TA, Klug JR, Silberman Y, Baucum AJ, Weitlauf C, Colbran RJ et al GluN2B subunit deletion reveals key role in acute and chronic ethanol sensitivity of glutamate synapses in bed nucleus of the stria terminalis. Proc Natl Acad Sci USA 2012; 109: E278–E287.
Regev L, Neufeld-Cohen A, Tsoory M, Kuperman Y, Getselter D, Gil S et al Prolonged and site-specific over-expression of corticotropin-releasing factor reveals differential roles for extended amygdala nuclei in emotional regulation. Mol Psychiatry 2011; 16: 714–728.
Kim SY, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS et al Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature 2013; 496: 219–223.
Brigman JL, Wright T, Talani G, Prasad-Mulcare S, Jinde S, Seabold GK et al Loss of GluN2B-containing NMDA receptors in CA1 hippocampus and cortex impairs long-term depression, reduces dendritic spine density, and disrupts learning. J Neurosci 2010; 30: 4590–4600.
Brewer JA, Khor B, Vogt SK, Muglia LM, Fujiwara H, Haegele KE et al T-cell glucocorticoid receptor is required to suppress COX-2-mediated lethal immune activation. Nat Med 2003; 9: 1318–1322.
Dulawa SC, Hen R . Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci Biobehav Rev 2005; 29: 771–783.
Keiser MJ, Setola V, Irwin JJ, Laggner C, Abbas AI, Hufeisen SJ et al Predicting new molecular targets for known drugs. Nature 2009; 462: 175–181.
Kolber BJ, Roberts MS, Howell MP, Wozniak DF, Sands MS, Muglia LJ . Central amygdala glucocorticoid receptor action promotes fear-associated CRH activation and conditioning. Proc Natl Acad Sci USA 2008; 105: 12004–12009.
Acknowledgements
This research was funded by NIH grants AA019455 (DGW) and MH079010 (LJM).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Translational Psychiatry website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Louderback, K., Wills, T., Muglia, L. et al. Knockdown of BNST GluN2B-containing NMDA receptors mimics the actions of ketamine on novelty-induced hypophagia. Transl Psychiatry 3, e331 (2013). https://doi.org/10.1038/tp.2013.103
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2013.103
Keywords
This article is cited by
-
BNST specific mGlu5 receptor knockdown regulates sex-dependent expression of negative affect produced by adolescent ethanol exposure and adult stress
Translational Psychiatry (2021)
-
Adolescent alcohol exposure produces sex differences in negative affect-like behavior and group I mGluR BNST plasticity
Neuropsychopharmacology (2020)
-
Optogenetic stimulation of medial prefrontal cortex Drd1 neurons produces rapid and long-lasting antidepressant effects
Nature Communications (2019)
-
Longitudinal two-photon imaging in somatosensory cortex of behaving mice reveals dendritic spine formation enhancement by subchronic administration of low-dose ketamine
Scientific Reports (2018)
-
Ketamine and its metabolite (2R,6R)-hydroxynorketamine induce lasting alterations in glutamatergic synaptic plasticity in the mesolimbic circuit
Molecular Psychiatry (2018)