Featured
-
-
-
-
Thesis |
 Tangible assets
Tangible assets
Michelle Francl argues we should embrace molecular models, not tuck them away in the closet.
- Michelle Francl
-
News & Views |
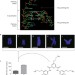 Visualizing the quadruplex
Visualizing the quadruplex
The direct observation and quantification of G-quadruplex structures formed from DNA in human cells during the cell cycle demonstrate the biological importance of these structures and point towards opportunities for targeting them with small-molecule drugs.
- Adam Siddiqui-Jain
- & Laurence H. Hurley
-
Article |
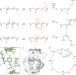 Volume-conserving trans–cis isomerization pathways in photoactive yellow protein visualized by picosecond X-ray crystallography
Volume-conserving trans–cis isomerization pathways in photoactive yellow protein visualized by picosecond X-ray crystallography
Time-resolved X-ray crystallography on photoactive yellow protein shows the existence of a short-lived, highly distorted intermediate whose reaction trajectory bifurcates along ‘bicycle-pedal’ and ‘hula-twist’ pathways. The bifurcating reaction pathways can be controlled by weakening the hydrogen bond between the chromophore and an adjacent residue, which switches off the bicycle-pedal pathway.
- Yang Ouk Jung
- , Jae Hyuk Lee
- & Hyotcherl Ihee
-
Article |
 Quantitative visualization of DNA G-quadruplex structures in human cells
Quantitative visualization of DNA G-quadruplex structures in human cells
A structure-specific antibody generated and employed to visualize DNA G-quadruplex structures in human cells shows that these structures are modulated during the cell cycle and can be stabilized by a small-molecule ligand. This provides substantive evidence for endogenous DNA G-quadruplex formation in mammalian cells.
- Giulia Biffi
- , David Tannahill
- & Shankar Balasubramanian
-
Article |
 Alteration of the oxygen-dependent reactivity of de novo Due Ferri proteins
Alteration of the oxygen-dependent reactivity of de novo Due Ferri proteins
Representing the first successful rational reprogramming of function in a de novo protein, the reactivity of a designed di-iron carboxylate protein from the Due Ferri family was altered from hydroquinone oxidation to arylamine N-hydroxylation through the introduction of a critical third histidine ligand in the active site.
- Amanda J. Reig
- , Marcos M. Pires
- & William F. DeGrado
-
Article |
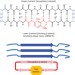 Amyloid β-sheet mimics that antagonize protein aggregation and reduce amyloid toxicity
Amyloid β-sheet mimics that antagonize protein aggregation and reduce amyloid toxicity
A family of robust β-sheet macrocycles that can display a variety of heptapeptide sequences from different amyloid proteins is introduced. These amyloid β-sheet mimics can be tailored to antagonize aggregation of the proteins, thereby reducing the toxicity associated with diseases such as Alzheimer's.
- Pin-Nan Cheng
- , Cong Liu
- & James S. Nowick
-
News & Views |
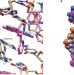 Into the minor groove
Into the minor groove
The interactions between ruthenium complexes and DNA duplexes, elucidated in detail in three different crystal structures, have been found to occur through the minor groove — an unexpected binding mode, but perhaps not such a strange one.
- Stephen Neidle
-
Article |
 Protein camouflage in cytochrome c–calixarene complexes
Protein camouflage in cytochrome c–calixarene complexes
A calixarene–protein host–guest complex has been characterized in detail by using a combination of NMR spectroscopy and X-ray crystallography. The water-soluble sulfonato-calix[4]arene binds to cytochrome c at various lysine residues to yield a dynamic complex. This interaction may serve to facilitate crystallization by mediating protein–protein contacts.
- Róise E. McGovern
- , Humberto Fernandes
- & Peter B. Crowley
-
News & Views |
 Mapping protein–protein contacts
Mapping protein–protein contacts
Obtaining detailed structural information about the interactions between amyloid-forming proteins and inhibitors can be extremely difficult. Two-dimensional infrared spectroscopy has now risen to this challenge to show the mapping of protein–protein contact sites in real time.
- Minhaeng Cho
-
Article |
 Darwinian evolution of an alternative genetic system provides support for TNA as an RNA progenitor
Darwinian evolution of an alternative genetic system provides support for TNA as an RNA progenitor
The pre-RNA-world hypothesis postulates that RNA was preceded in the evolution of life by a simpler genetic material. Here, Darwinian evolution methods were used to generate a threose nucleic acid (TNA) aptamer. This result provides evidence that TNA could have served as an ancestral genetic system during an early stage of life.
- Hanyang Yu
- , Su Zhang
- & John C. Chaput
-
News & Views |
 Adding a second dimension
Adding a second dimension
Mutating RNA one nucleotide at a time and measuring the impact of this on its chemical reactivity provides a strategy for determining its three-dimensional structure, and from there, hopefully, its function.
- Katja Petzold
- & Hashim M. Al-Hashimi
-
Article |
 A two-dimensional mutate-and-map strategy for non-coding RNA structure
A two-dimensional mutate-and-map strategy for non-coding RNA structure
Non-coding RNAs are ubiquitous biomolecules with intricate three-dimensional folds that are difficult to characterize. This Article presents an information-rich strategy for inferring RNA structure by combining nucleotide-by-nucleotide mutagenesis with single-nucleotide-resolution chemical mapping.
- Wipapat Kladwang
- , Christopher C. VanLang
- & Rhiju Das
-
News & Views |
 Spinning into focus
Spinning into focus
Transient sedimentation of proteins inside a solid-state NMR rotor under fast magic-angle spinning offers a promising solution to the challenge of determining the structures of high-molecular-weight proteins with atomic resolution. This opens new opportunities for structural analysis of large macromolecules and macromolecular assemblies.
- Tatyana Polenova
-
Article |
 Reversible bond formation enables the replication and amplification of a crosslinking salen complex as an orthogonal base pair
Reversible bond formation enables the replication and amplification of a crosslinking salen complex as an orthogonal base pair
Adding one further base pair to the classic Watson–Crick scheme not only expands the genetic code but also offers opportunities to modify the structure and function of DNA. It has now been shown that an artificial metal–salen base pair can be enzymatically incorporated into DNA duplexes and even amplified by PCR.
- Corinna Kaul
- , Markus Müller
- & Thomas Carell
-
Article |
 Ion mobility–mass spectrometry reveals a conformational conversion from random assembly to β-sheet in amyloid fibril formation
Ion mobility–mass spectrometry reveals a conformational conversion from random assembly to β-sheet in amyloid fibril formation
Amyloid cascades leading to peptide β-sheet fibrils are central to many diseases. Intermediate assemblies were recently identified as the toxic agents, but obtaining structural details of these early oligomers has largely been unsuccessful with traditional techniques. Here, ion mobility methods provide evidence for structural transitions from random to β-sheet assembly.
- Christian Bleiholder
- , Nicholas F. Dupuis
- & Michael T. Bowers
-
Article |
 Interrogating viral capsid assembly with ion mobility–mass spectrometry
Interrogating viral capsid assembly with ion mobility–mass spectrometry
Although most proteins fulfil their role as part of large protein complexes, little is known about the pathways of complex assembly. Here, ion mobility–mass spectrometry is used to monitor and structurally characterize the assembly intermediates of viral protein shells, called capsids, of two major human pathogens, norovirus and hepatitis B virus.
- Charlotte Uetrecht
- , Ioana M. Barbu
- & Albert J. R. Heck
-
News & Views |
 Control of active-site compression
Control of active-site compression
Compression of the active sites of enzymes has been linked to the bulk of amino acid side chains, but now experiments highlight that the harder we look, the more curious the relationship between protein structure and function becomes.
- Judith P. Klinman
-
Article |
 Crystallographic snapshots of the reaction of aromatic C–H with O2 catalysed by a protein-bound iron complex
Crystallographic snapshots of the reaction of aromatic C–H with O2 catalysed by a protein-bound iron complex
Crystallographically studying chemical reactions is difficult because the intermediates are rarely stable enough to be crystallized. Now, protein crystals have been used to trap four reaction intermediates in an aromatic dihydroxylation, showing that an iron peroxo complex is observed.
- Christine Cavazza
- , Constance Bochot
- & Stéphane Ménage
-
Research Highlights |
 Under a graphene cover
Under a graphene cover
Layers of water adsorbed on a mica surface have been trapped under a graphene sheet and their structure determined by atomic force microscopy.
- Anne Pichon
-
News & Views |
 A new phase for protein chemistry
A new phase for protein chemistry
Proteins are most at home in water, although it has been known for some time that they can remain functional in non-aqueous environments. Researchers have now shown that in solvent-free melts, the oxygen-binding protein myoglobin adopts a near-native structure and retains its biological activity.
- Douglas S. Clark
-
Article |
 Crystal structure of a metal ion-bound oxoiron(IV) complex and implications for biological electron transfer
Crystal structure of a metal ion-bound oxoiron(IV) complex and implications for biological electron transfer
The interactions of metal ions with metaloxo species are crucial in many important biological processes, such as the oxygen-evolving complex (OEC) in photosystem II, but their exact function remains elusive. Now, the binding of metal ions to a non-haem oxoiron complex has been studied and the observed changes to its electron-transfer properties provide insights into the active site of the OEC.
- Shunichi Fukuzumi
- , Yuma Morimoto
- & Wonwoo Nam
-
Article |
 Reversible dioxygen binding in solvent-free liquid myoglobin
Reversible dioxygen binding in solvent-free liquid myoglobin
Freeze-drying of aqueous myoglobin–polymer surfactant nanoconjugates affords a water-free solid that melts at room temperature to produce a viscous solventless liquid protein that exhibits near-native secondary structure and reversible dioxygen binding. The results challenge the accepted role of solvent molecules in mediating protein structure and function, and offer new opportunities in protein-based nanoscience and bionanotechnology.
- Adam W. Perriman
- , Alex P. S. Brogan
- & Stephen Mann
-
Research Highlights |
 X-ray ordering
X-ray ordering
Exposing supramolecular filaments to X-rays results in spontaneous and reversible crystalline ordering.
- Neil Withers
-

 Probing the biophysical interplay between a viral genome and its capsid
Probing the biophysical interplay between a viral genome and its capsid Crowning achievement
Crowning achievement Tomography reveals all
Tomography reveals all