Featured
-
-
Article
| Open Access Direct catalytic asymmetric synthesis of α-chiral bicyclo[1.1.1]pentanes
Direct catalytic asymmetric synthesis of α-chiral bicyclo[1.1.1]pentanes
Bicyclo[1.1.1]pentanes (BCPs) are important motifs in contemporary drug design, however, approaches to BCPs featuring adjacent stereocenters are rather limited. Here, the authors report a photo- and organocatalyzed asymmetric addition of simple aldehydes to [1.1.1]propellane to generate enantioenriched α-chiral BCPs.
- Marie L. J. Wong
- , Alistair J. Sterling
- & Edward A. Anderson
-
Article
| Open Access Catalytic asymmetric reductive hydroalkylation of enamides and enecarbamates to chiral aliphatic amines
Catalytic asymmetric reductive hydroalkylation of enamides and enecarbamates to chiral aliphatic amines
Enantiopure aliphatic amines are frequently encountered as chiral auxiliaries and synthetic intermediates for bioactive compounds. Here, the authors report a mild nickel-catalysed asymmetric reductive hydroalkylation to convert enamides and enecarbamates into α-branched chiral amines and derivatives.
- Jia-Wang Wang
- , Yan Li
- & Yao Fu
-
Article
| Open Access Cavity frequency-dependent theory for vibrational polariton chemistry
Cavity frequency-dependent theory for vibrational polariton chemistry
Vibrational strong coupling controls the ground-state reactivity of molecules in optical cavities, but the underlying theory is still elusive. The authors analyze a molecular system coupled to a cavity mode and find that the reaction rate is suppressed for a particular cavity frequency, related to the topology of the reaction barrier region, analogously to a solvent caging effect.
- Xinyang Li
- , Arkajit Mandal
- & Pengfei Huo
-
Article
| Open Access Gold(I)-catalyzed intramolecular cyclization/intermolecular cycloaddition cascade as a fast track to polycarbocycles and mechanistic insights
Gold(I)-catalyzed intramolecular cyclization/intermolecular cycloaddition cascade as a fast track to polycarbocycles and mechanistic insights
Metal carbene is usually employed as a 1-carbon synthon or 3-carbon synthon in a variety of cycloaddition reactions. Here, the authors report a gold-catalyzed cascade protocol for the assembly of polycarbocyclic frameworks via a β-aryl gold-carbene intermediate which reacts as a 4-carbon synthon with alkenes in [4 + 2]-cycloadditions.
- Cheng Zhang
- , Kemiao Hong
- & Xinfang Xu
-
Article
| Open Access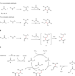 Three-component radical homo Mannich reaction
Three-component radical homo Mannich reaction
Due to the ionic nature of its mechanism, the Mannich reaction can only use non-enolizable aldehydes as substrates. Here, the authors expand the scope of the classical Mannich reaction to enolizable aldehydes by employing a radical process resulting in a streamlined synthesis of γ-amino-carbonyl compounds.
- Shuai Shi
- , Wenting Qiu
- & Zhankui Sun
-
Article
| Open Access Electronic spin separation induced by nuclear motion near conical intersections
Electronic spin separation induced by nuclear motion near conical intersections
Spin polarization is at the basis of quantum information and underlies some natural processes, but many aspects still need to be explored. Here, the authors, by quantum mechanical computations, show that even a weak spin-orbit coupling near a conical intersection can induce large spin selection, with consequences for spin manipulation in photochemical or electrochemical reactions.
- Yanze Wu
- & Joseph E. Subotnik
-
Article
| Open Access Passerini-type reaction of boronic acids enables α-hydroxyketones synthesis
Passerini-type reaction of boronic acids enables α-hydroxyketones synthesis
Multicomponent reactions enable the rapid construction of diverse molecular scaffolds with modularity and step economy. In this work, the authors report the use of boronic acids as carbon nucleophiles in a Passerini-type three-component coupling reaction towards an expanded inventory of α-hydroxyketones.
- Kai Yang
- , Feng Zhang
- & Qiuling Song
-
Article
| Open Access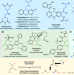 Vicinal difunctionalization of carbon–carbon double bond for the platform synthesis of trifluoroalkyl amines
Vicinal difunctionalization of carbon–carbon double bond for the platform synthesis of trifluoroalkyl amines
Metal-free olefin diamination may display challenges, especially when targeting fluorinated amines. Here, the authors report a double nucleophilic functionalization of an activated alkene originated from a trifluoropropenyliodonium salt with two nucleophiles for the selective synthesis of trifluoromethylated ethylene amines and diamines.
- Ferenc Béke
- , Ádám Mészáros
- & Zoltán Novák
-
Article
| Open Access Alkyl halides as both hydride and alkyl sources in catalytic regioselective reductive olefin hydroalkylation
Alkyl halides as both hydride and alkyl sources in catalytic regioselective reductive olefin hydroalkylation
Methods that regioselectively install a functionalized alkyl unit at the 2-position of a terminal unactivated C=C bond are scarce. Here, the authors report a Markovnikov-selective hydroalkylation of unactivated amide-tethered alkenes catalyzed by nickel in conjunction with a stoichiometric reductant.
- Xianxiao Chen
- , Weidong Rao
- & Ming Joo Koh
-
Article
| Open Access Complex reaction processes in combustion unraveled by neural network-based molecular dynamics simulation
Complex reaction processes in combustion unraveled by neural network-based molecular dynamics simulation
Gaining insights into combustion processes is challenging due to the complex reactions involved. The present work proposes a neural network potential model trained to ab initio data that enables to simulate the combustion of methane by predicting reactants, products and reaction intermediates.
- Jinzhe Zeng
- , Liqun Cao
- & John Z. H. Zhang
-
Article
| Open Access Skeletal reorganization divergence of N-sulfonyl ynamides
Skeletal reorganization divergence of N-sulfonyl ynamides
Skeletal reorganizations are intriguing processes in chemical synthesis due to their mechanism, atom-economy and synthetic versatility. Herein, the authors describe a divergent skeletal reorganization of N-sulfonyl ynamides to thiete sulfones and propargyl sulfonamides.
- Linwei Zeng
- , Yuxin Lin
- & Sunliang Cui
-
Article
| Open Access State-of-the-art augmented NLP transformer models for direct and single-step retrosynthesis
State-of-the-art augmented NLP transformer models for direct and single-step retrosynthesis
Development of algorithms to predict reactant and reagents given a target molecule is key to accelerate retrosynthesis approaches. Here the authors demonstrate that applying augmentation techniques to the SMILE representation of target data significantly improves the quality of the reaction predictions.
- Igor V. Tetko
- , Pavel Karpov
- & Guillaume Godin
-
Article
| Open Access Chiral phosphoric acid-catalyzed stereodivergent synthesis of trisubstituted allenes and computational mechanistic studies
Chiral phosphoric acid-catalyzed stereodivergent synthesis of trisubstituted allenes and computational mechanistic studies
Despite of the high demand of chiral allenes, their asymmetric synthesis remains a challenge for organic chemists. Here, the authors report a stereodivergent synthesis of trisubstituted allenes via asymmetric additions of oxazolones to activated 1,3-enynes enabled by modification of chiral phosphoric acid catalysts.
- Jiawen Wang
- , Sujuan Zheng
- & Xiaoyu Yang
-
Article
| Open Access Decarboxylative thiolation of redox-active esters to free thiols and further diversification
Decarboxylative thiolation of redox-active esters to free thiols and further diversification
Thiols are important precursors for the synthesis of a variety of pharmaceutically important sulfur-containing compounds. Here, the authors report a visible light-mediated decarboxylative thiolation of alkyl redox-active esters to free thiols and the in situ product diversification of a number of thiol derivatives.
- Tianpeng Cao
- , Tianxiao Xu
- & Saihu Liao
-
Article
| Open Access Cobalt-catalyzed deoxygenative triborylation of allylic ethers to access 1,1,3-triborylalkanes
Cobalt-catalyzed deoxygenative triborylation of allylic ethers to access 1,1,3-triborylalkanes
Organoboron compounds are versatile intermediates in chemical synthesis. Here, the authors report a selective cobalt-catalyzed deoxygenative triborylation of allylic ethers with pinacolborane to prepare 1,1,3-triborylalkane compounds.
- Wei Jie Teo
- , Xiaoxu Yang
- & Shaozhong Ge
-
Article
| Open Access Manganese-mediated reductive functionalization of activated aliphatic acids and primary amines
Manganese-mediated reductive functionalization of activated aliphatic acids and primary amines
Alkyl carboxylic acids and primary amines are ubiquitous and useful for synthesis of new compounds. Here, the authors report a manganese-mediated reductive decarboxylative/deaminative functionalization of activated aliphatic acids and primary amines for construction of C-C and C-X bonds under mild conditions.
- Zhan Li
- , Ke-Feng Wang
- & Honggen Wang
-
Article
| Open Access Enhanced polymer mechanical degradation through mechanochemically unveiled lactonization
Enhanced polymer mechanical degradation through mechanochemically unveiled lactonization
The mechanical degradation of polymers is typically limited to a single chain scission event and the loss of stress transfer during the scission process limits the extent of degradation achieved. Here, the authors report a mechanically triggered, delayed scission strategy that allows many eventual scission events to be initiated within a single polymer chain.
- Yangju Lin
- , Tatiana B. Kouznetsova
- & Stephen L. Craig
-
Article
| Open Access A robust and tunable halogen bond organocatalyzed 2-deoxyglycosylation involving quantum tunneling
A robust and tunable halogen bond organocatalyzed 2-deoxyglycosylation involving quantum tunneling
Halogen bonding (HB) catalysis is rapidly gaining momentum, however, cases of XB activation for challenging bonds formation are rare. Here, the authors show a robust XB catalyzed 2-deoxyglycosylation with broad scope and featuring a quantum tunneling phenomenon in the proton transfer rate determining step.
- Chunfa Xu
- , V. U. Bhaskara Rao
- & Charles C. J. Loh
-
Article
| Open Access Conversion of anilines to chiral benzylic amines via formal one-carbon insertion into aromatic C–N bonds
Conversion of anilines to chiral benzylic amines via formal one-carbon insertion into aromatic C–N bonds
Atom insertion into aromatic carbon-nitrogen (C-N) bonds is useful for the synthesis of nitrogen-containing molecules, but challenging due to the inert nature of these bonds. Here, the authors report one-carbon insertion into aromatic C-N bonds to directly convert anilines to chiral benzylic amines.
- Lei Li
- , Min Yang
- & Renhua Fan
-
Article
| Open Access Ring-opening functionalizations of unstrained cyclic amines enabled by difluorocarbene transfer
Ring-opening functionalizations of unstrained cyclic amines enabled by difluorocarbene transfer
Cyclic amines are commonly present in natural products and synthetic compounds, but methods for their skeletal diversification are limited. Here, the authors report a strategy for selective ring-opening functionalization of unstrained cyclic amines to pluripotent products that can be further diversified.
- Youyoung Kim
- , Joon Heo
- & Sangwon Seo
-
Article
| Open Access Addition of alkynes and osmium carbynes towards functionalized dπ–pπ conjugated systems
Addition of alkynes and osmium carbynes towards functionalized dπ–pπ conjugated systems
Metal-carbon and carbon-carbon triple bonds are highly unsaturated bonds and their reactions tend to afford cycloaddition intermediates or products. Here, the authors report a new robust reaction on metal-carbon and carbon-carbon triple bonds which affords region- and stereospecific acyclic addition products.
- Shiyan Chen
- , Longzhu Liu
- & Haiping Xia
-
Comment
| Open Access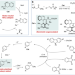 Spotting trends in organocatalysis for the next decade
Spotting trends in organocatalysis for the next decade
After two decades of steady growing, symbiotic merger of organocatalysis with emerging electrochemical and photochemical tools are envisioned as hot topics in the coming decade. Here, these trends are discussed in parallel to the implementation of artificial intelligence-based technologies, which anticipate a paradigm shift in catalyst design.
- José M. Lassaletta
-
Article
| Open Access Observation of the geometric phase effect in the H+HD→H2+D reaction below the conical intersection
Observation of the geometric phase effect in the H+HD→H2+D reaction below the conical intersection
The geometric phase effect associated with a conical intersection between the ground and first excited electronic state has been predicted in the H3 system below the conical intersection energy. The authors, by a crossed molecular beam technique and quantum dynamic calculations, provide experimental evidence and insight into its origin.
- Daofu Yuan
- , Yin Huang
- & Xueming Yang
-
Article
| Open Access Deazaflavin reductive photocatalysis involves excited semiquinone radicals
Deazaflavin reductive photocatalysis involves excited semiquinone radicals
Flavins and deazaflavins are well suited for photoredox processes but their application in photoreductions is challenging. Here, the authors provide direct evidence of the high reductive power of excited deazaflavin semiquinones and their application in catalytic photodehalogenations.
- Andreas Graml
- , Tomáš Neveselý
- & Burkhard König
-
Article
| Open Access Transition-metal-free formal cross-coupling of aryl methyl sulfoxides and alcohols via nucleophilic activation of C-S bond
Transition-metal-free formal cross-coupling of aryl methyl sulfoxides and alcohols via nucleophilic activation of C-S bond
Cross-coupling processes without the use of transition metals are challenging to achieve. Here, the authors show a transition-metal-free cross-coupling utilizing aryl(heteroaryl) methyl sulfoxides and alcohols to afford alkyl aryl(heteroaryl) ethers and propose a nucleophilic addition mechanism based on experiments and theory.
- Guolin Li
- , Yexenia Nieves-Quinones
- & Tiezheng Jia
-
Article
| Open Access Direct transfer of tri- and di-fluoroethanol units enabled by radical activation of organosilicon reagents
Direct transfer of tri- and di-fluoroethanol units enabled by radical activation of organosilicon reagents
Methods for direct incorporation of tri- and di-fluoroethanol units in molecules are relatively limited. Here, the authors report two organosilicon reagents which are applied to allylation, alkylation and alkenylation reactions as tri- and di-fluoroethanol transfer reagents.
- Xiang Chen
- , Xingxing Gong
- & Xiao Shen
-
Article
| Open Access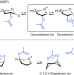 Characterization of glycosyl dioxolenium ions and their role in glycosylation reactions
Characterization of glycosyl dioxolenium ions and their role in glycosylation reactions
Dioxolenium ion intermediates formed from remote positions are hypothesized to direct stereoselective glycosylations. Herein we combine infrared ion spectroscopy, DFT calculations and synthetic work to characterize and study these dioxolenium ions and their role in stereoselective glycosylation reactions.
- Thomas Hansen
- , Hidde Elferink
- & Thomas J. Boltje
-
Article
| Open Access Integrated redox-active reagents for photoinduced regio- and stereoselective fluorocarboborylation
Integrated redox-active reagents for photoinduced regio- and stereoselective fluorocarboborylation
The synthesis of vinylboronates and alkylboronates often suffers from step-tedious and poorly stereoselective procedures. Here, the authors report a bench-stable redox-active reagent for the radical difunctionalization of alkenes and alkynes affording fluorine-containing vinylboronates and alkylboronates.
- Weigang Zhang
- , Zhenlei Zou
- & Yi Pan
-
Article
| Open Access Transient-axial-chirality controlled asymmetric rhodium-carbene C(sp2)-H functionalization for the synthesis of chiral fluorenes
Transient-axial-chirality controlled asymmetric rhodium-carbene C(sp2)-H functionalization for the synthesis of chiral fluorenes
The formation of chiral molecules generally relies on direct chirality transfer from catalyst to products. Here, the authors report a strategy based on point chirality transfer from the catalyst to a dirhodium carbene intermediate with axial chirality, which is then transferred to products via C(sp2)-H functionalization.
- Kuiyong Dong
- , Xing Fan
- & Xinfang Xu
-
Article
| Open Access Room-temperature chemical synthesis of C2
Room-temperature chemical synthesis of C2
Diatomic carbon (C2) is historically an elusive chemical species, considered to require high physical energy for its generation. Here, the authors describe the first room-temperature chemical synthesis of C2 and present experimental evidence for its singlet biradical (quadruple bonding) character and role as a molecular element of nanocarbons.
- Kazunori Miyamoto
- , Shodai Narita
- & Masanobu Uchiyama
-
Article
| Open Access Unusual KIE and dynamics effects in the Fe-catalyzed hetero-Diels-Alder reaction of unactivated aldehydes and dienes
Unusual KIE and dynamics effects in the Fe-catalyzed hetero-Diels-Alder reaction of unactivated aldehydes and dienes
Recently an iron catalyst was developed to catalyze an oxa-Diels-Alder reaction, whose mechanism is unclear yet. Here the authors combine DFT and molecular dynamics simulations with experimental studies to elucidate the unusual iron effect on kinetic isotope effect and dynamics in this reaction.
- Yuhong Yang
- , Xiaoyong Zhang
- & Lung Wa Chung
-
Article
| Open Access Visible-light promoted regioselective amination and alkylation of remote C(sp3)-H bonds
Visible-light promoted regioselective amination and alkylation of remote C(sp3)-H bonds
C-N bond forming is an established strategy to form amines, which are quintessential in chemical synthesis and in nature. Here, the authors report three classes of photoredox reactions, involving C(sp3)-N coupling between N-centered radicals and alkyl radicals and C(sp3)- C(sp3) coupling via C(sp3)-H alkylation.
- Quanping Guo
- , Qiang Peng
- & Zhaoqing Xu
-
Article
| Open Access Intermolecular [3+3] ring expansion of aziridines to dehydropiperi-dines through the intermediacy of aziridinium ylides
Intermolecular [3+3] ring expansion of aziridines to dehydropiperi-dines through the intermediacy of aziridinium ylides
Traditional synthesis of stereodefined piperidines requires selective installation of functional groups that can lower efficiency and modularity. Here, the authors assemble stereochemically complex and highly substituted dehydropiperidines via an intermolecular ring expansion between bicyclic aziridines and Rh-supported vinyl carbenes.
- Josephine Eshon
- , Kate A. Nicastri
- & Jennifer M. Schomaker
-
Article
| Open Access A unified approach for divergent synthesis of contiguous stereodiads employing a small boronyl group
A unified approach for divergent synthesis of contiguous stereodiads employing a small boronyl group
Predictable and unified approaches to all possible stereoisomers of acyclic compounds with contiguous stereocentres are rare. Here, the authors disclose a divergent α-functionalization of enolates with either syn or anti selectivity employing a β-boronyl group as a small, directing handle.
- Miao Zhan
- , Zhengwei Ding
- & Pengfei Li
-
Article
| Open Access Nucleophilic trifluoromethoxylation of alkyl halides without silver
Nucleophilic trifluoromethoxylation of alkyl halides without silver
Trifluoromethyl ethers are important bioactive targets in pharmaceuticals and agrochemicals, however, their synthesis is often not straightforward. Here, the authors disclose a reagent for the nucleophilic trifluoromethoxylation of alkyl halides without silver and under mild conditions.
- Yan Li
- , Yang Yang
- & Pingping Tang
-
Article
| Open Access Radical-mediated C-C cleavage of unstrained cycloketones and DFT study for unusual regioselectivity
Radical-mediated C-C cleavage of unstrained cycloketones and DFT study for unusual regioselectivity
C-C bond scission of unstrained cycloketones with high regioselectivity is a challenging synthetic task. Here, the authors show a facile C-C cleavage of cyclohexanones and cyclopentanones with unusual selectivity under mild conditions with the aid of an in situ formed side-chain aryl radical.
- Mingyang Wang
- , Man Li
- & Chen Zhu
-
Article
| Open Access Interplay of water and a supramolecular capsule for catalysis of reductive elimination reaction from gold
Interplay of water and a supramolecular capsule for catalysis of reductive elimination reaction from gold
Supramolecular catalytic assemblies attract enormous interest due to their activity that rivals natural enzymes. Using ab initio molecular dynamics, the authors show that a gold catalyst in a Ga4L612- nanocage, while impeded by reorganization energy, is accelerated by hosting a catalytic water molecule.
- Valerie Vaissier Welborn
- , Wan-Lu Li
- & Teresa Head-Gordon
-
Article
| Open Access Synthesis of difluoromethylated allenes through trifunctionalization of 1,3-enynes
Synthesis of difluoromethylated allenes through trifunctionalization of 1,3-enynes
Fluorinated or fluoroalkylated allenes are versatile building blocks for medicinal and material chemistry. Here, the authors show a regioselective trifunctionalization of 1,3-enynes proceeding through double C-F bond formation and concomitant installation of a -NSO2Ph group to the allene moiety.
- Munira Taj Muhammad
- , Yihang Jiao
- & Hongli Bao
-
Article
| Open Access Functionalization of remote C(sp3)-H bonds enabled by copper-catalyzed coupling of O-acyloximes with terminal alkynes
Functionalization of remote C(sp3)-H bonds enabled by copper-catalyzed coupling of O-acyloximes with terminal alkynes
Sonogashira cross-coupling is a powerful strategy to prepare functionalized internal alkynes. Here, the authors report a domino sequence for the generation of γ-/δ-alkynyl nitriles and γ-alkynyl ketones from the coupling of terminal alkynes with O-acyloximes derived from cycloalkanones and acylic ketones, respectively.
- Zhaodong Li
- , Rubén O. Torres-Ochoa
- & Jieping Zhu
-
Article
| Open Access Reaction scope and mechanistic insights of nickel-catalyzed migratory Suzuki–Miyaura cross-coupling
Reaction scope and mechanistic insights of nickel-catalyzed migratory Suzuki–Miyaura cross-coupling
Migratory cross-coupling reactions are powerful tools to form bonds at predictable positions. Here the authors report a nickel-catalyzed migratory Suzuki–Miyaura cross-coupling of unactivated alkyl electrophiles with aryl and vinyl boron reagents and provide experimental and computational mechanistic evidence.
- Yuqiang Li
- , Yixin Luo
- & Guoyin Yin
-
Article
| Open Access Feshbach resonances in the F + H2O → HF + OH reaction
Feshbach resonances in the F + H2O → HF + OH reaction
Feshbach resonances are transiently trapped states along a reaction coordinate, providing a probe to the reaction’s potential energy surface (PES) but difficult to analyze in polyatomic systems. Here the authors identify Feshbach resonances in a reacting 4-atom system by state-to-state quantum dynamics using a full-dimensional PES.
- Xiaoren Zhang
- , Lulu Li
- & Dong H. Zhang
-
Article
| Open Access Acyl radical to rhodacycle addition and cyclization relay to access butterfly flavylium fluorophores
Acyl radical to rhodacycle addition and cyclization relay to access butterfly flavylium fluorophores
Structural diversity of organic fluorophores is of importance for several applications (fluorescent markers, photosensitizers, etc.). Here the authors report a method to merge radical chemistry with C–H activation to construct a brand-new class of flavylium fluorophores starting from (hetero)aryl ketones and alkynes.
- Jiangliang Yin
- , Yuming Zhang
- & Jingsong You
-
Article
| Open Access Hyperconjugative aromaticity and protodeauration reactivity of polyaurated indoliums
Hyperconjugative aromaticity and protodeauration reactivity of polyaurated indoliums
Hyperconjugative aromaticity combines the concepts of hyperconjugation and aromaticity and explains cyclopentadiene stability. Here, the authors demonstrate extended hyperconjugative aromaticity in a metallated indole ring, which shows extended electron conjugation due to the dual hyperconjugation.
- Kui Xiao
- , Yu Zhao
- & Liang Zhao
-
Article
| Open Access Solid-to-liquid phase transitions of sub-nanometer clusters enhance chemical transformation
Solid-to-liquid phase transitions of sub-nanometer clusters enhance chemical transformation
Understanding the dynamic evolution of the catalysts’ structure under reaction conditions is crucial in heterogeneous catalysis. Here the authors use ab initio molecular dynamics simulations to show an anomalous decrease in reaction free energies and barriers on dynamical sub-nanometer Au clusters supported on MgO(001).
- Juan-Juan Sun
- & Jun Cheng
-
Article
| Open Access Oxidation of difluorocarbene and subsequent trifluoromethoxylation
Oxidation of difluorocarbene and subsequent trifluoromethoxylation
Difluorocarbene is a versatile and efficient intermediate for fluorine incorporation. Here, the authors show that difluorocarbene can be oxidized to carbonyl fluoride and this process is confirmed in 18O-trifluoromethoxylation reactions, by observation of AgOCF3 species and theory.
- Jiao Yu
- , Jin-Hong Lin
- & Ji-Chang Xiao
-
Article
| Open Access An umpolung strategy to react catalytic enols with nucleophiles
An umpolung strategy to react catalytic enols with nucleophiles
Nucleophiles cannot be directly reacted with enolates due to polarity mismatching. Here, the authors developed an umpolung strategy for the selective synthesis of α-alkoxy carbonyl compounds by reaction of iridium enolates with nucleophilic alcohols promoted by an iodine(III) reagent.
- Amparo Sanz-Marco
- , Samuel Martinez-Erro
- & Belén Martín-Matute
-
Article
| Open Access Site-selective remote C(sp3)–H heteroarylation of amides via organic photoredox catalysis
Site-selective remote C(sp3)–H heteroarylation of amides via organic photoredox catalysis
Nitrogen-centered radicals are valuable intermediates for the controllable and selective functionalization of remote inert C(sp3)–H bonds. Here, the authors report an amidyl radical-triggered, metal-free and site-selective C(sp3)–H heteroarylation of amides under photoredox conditions.
- Hui Chen
- , Wenjing Fan
- & Shouyun Yu
-
Article
| Open Access Resonant catalysis of thermally activated chemical reactions with vibrational polaritons
Resonant catalysis of thermally activated chemical reactions with vibrational polaritons
Strong coupling of molecular vibrations to an optical cavity may catalyze thermally activated reactions, showcasing the potential of polariton chemistry. Here, the authors provide a theoretical framework explaining the chemical kinetics deriving from transit through polaritonic and dark states.
- Jorge A. Campos-Gonzalez-Angulo
- , Raphael F. Ribeiro
- & Joel Yuen-Zhou
-
Article
| Open Access Modular synthesis of α-fluorinated arylmethanes via desulfonylative cross-coupling
Modular synthesis of α-fluorinated arylmethanes via desulfonylative cross-coupling
α-Fluoromethyl arenes are common substructures in pharmaceuticals and agrochemicals but synthetic routes are still rare. Here the authors describe Pd-catalyzed Suzuki-Miyaura cross-coupling of α-fluorinated benzylic triflones with arylboronic acids affording structurally diverse α-fluorinated diarylmethanes.
- Masakazu Nambo
- , Jacky C.-H. Yim
- & Cathleen M. Crudden

 Asymmetric three-component olefin dicarbofunctionalization enabled by photoredox and copper dual catalysis
Asymmetric three-component olefin dicarbofunctionalization enabled by photoredox and copper dual catalysis