News & Views |
Featured
-
-
News & Views |
 Thread and cut
Thread and cut
Processive catalysis is frequent in nature, but much less common in synthetic systems. Now, a semisynthetic biohybrid catalytic system is reported that oxidizes DNA in a processive manner.
- Leonard J. Prins
- & Paolo Scrimin
-
Article |
 A thiamin-utilizing ribozyme decarboxylates a pyruvate-like substrate
A thiamin-utilizing ribozyme decarboxylates a pyruvate-like substrate
Vitamins are thought to be relics of a primordial RNA World. A demonstration that catalytic RNAs are capable of harnessing vitamin cofactors would support the likely role of vitamins in early metabolic processes. Here, a ribozyme that uses vitamin B1 to aid decarboxylation of a pyruvate-like substrate is reported.
- Paul Cernak
- & Dipankar Sen
-
Article |
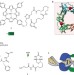 A clamp-like biohybrid catalyst for DNA oxidation
A clamp-like biohybrid catalyst for DNA oxidation
Clamp proteins that encircle DNA and then recruit enzymes are one of nature's ways of making catalysis on DNA processive. Here, a clamp protein is equipped with a synthetic catalyst that sequence-specifically oxidizes DNA. The resulting biohybrid catalyst shows processive behaviour, which is visualized by atomic force microscopy.
- Stijn F. M. van Dongen
- , Joost Clerx
- & Roeland J. M. Nolte
-
News & Views |
 Proteins in a pill
Proteins in a pill
Protein drugs are important therapies for many different diseases, but very few can be administered orally. Now, a cationic dendronized polymer has been shown to stabilize a therapeutic protein for delivery to the gut.
- Heather D. Maynard
-
Article |
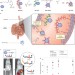 Sustained gastrointestinal activity of dendronized polymer–enzyme conjugates
Sustained gastrointestinal activity of dendronized polymer–enzyme conjugates
Methods for stabilizing enzymatic activity in the gastrointestinal tract are rarely investigated because of the difficulty in protecting proteins from an environment that promotes their digestion. Now, functionally diverse polymers have been conjugated to therapeutic enzymes, which lead to a substantial enhancement of their in vivo activity in the gastrointestinal tract.
- Gregor Fuhrmann
- , Andrea Grotzky
- & Jean-Christophe Leroux
-
Article |
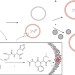 Competition between model protocells driven by an encapsulated catalyst
Competition between model protocells driven by an encapsulated catalyst
Darwinian evolution involves competition between members of a population. Here, the synthesis of a hydrophobic dipeptide catalysed by a second dipeptide in a model protocell — a vesicle — is described. The reaction product partitions to the vesicle membrane, which grows by accumulating fatty acids derived from neighbouring vesicles. Thus, an encapsulated catalyst drives competition between the model protocells.
- Katarzyna Adamala
- & Jack W. Szostak
-
-
Article |
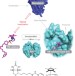 Synthetic cascades are enabled by combining biocatalysts with artificial metalloenzymes
Synthetic cascades are enabled by combining biocatalysts with artificial metalloenzymes
An artificial transfer hydrogenase, based on the incorporation of a biotinylated iridium-piano-stool complex in streptavidin, is shown to be fully compatible with a range of biocatalysts. The location of the active metal centre inside the protein scaffold efficiently prevents mutual inactivation processes and enables the concurrent interplay with oxidative enzymes.
- V. Köhler
- , Y. M. Wilson
- & T. R. Ward
-
News & Views |
 Engineering di-iron enzymes
Engineering di-iron enzymes
A dramatic switch of reactivity — from hydroquinone oxidation to N-hydroxylation — can be achieved through the rational engineering of a de novo-designed di-iron protein. Four specific amino-acid mutations spread throughout the first, second and third coordination shells result in a million-fold increase in the relative rate of these two reactions.
- Steven M. Berry
-
News & Views |
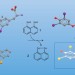 Halogen and chalcogen team up
Halogen and chalcogen team up
The behaviour of di-selenol enzyme mimics indicates that a halogen bond between selenium and iodine, and a chalcogen interaction between the two selenium atoms, play an important role in the activation of thyroid hormones.
- Pierangelo Metrangolo
- & Giuseppe Resnati
-
Article |
 Engineering methylaspartate ammonia lyase for the asymmetric synthesis of unnatural amino acids
Engineering methylaspartate ammonia lyase for the asymmetric synthesis of unnatural amino acids
Substituted aspartic acids are highly valuable as tools for biological research and as chiral building blocks for pharmaceuticals. Here, engineering of the enzyme methylaspartate ammonia lyase to accept a large variety of substituted amines and fumarates and catalyse the asymmetric synthesis of aspartic acid derivatives is described.
- Hans Raj
- , Wiktor Szymański
- & Gerrit J. Poelarends
-
Article |
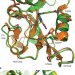 Evidence that a ‘dynamic knockout’ in Escherichia coli dihydrofolate reductase does not affect the chemical step of catalysis
Evidence that a ‘dynamic knockout’ in Escherichia coli dihydrofolate reductase does not affect the chemical step of catalysis
The connection between protein dynamics and catalysis is an issue of vigorous debate in enzymology. Conformational motions are known to be important for the physical steps in the catalytic cycle of dihydrofolate reductase, however, it is now reported that there is no evidence of a correlation between such motions and the actual chemical step, hydride transfer.
- E. Joel Loveridge
- , Enas M. Behiry
- & Rudolf K. Allemann
-
Perspective |
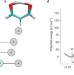 Good vibrations in enzyme-catalysed reactions
Good vibrations in enzyme-catalysed reactions
This Perspective discusses contemporary ideas for enzymatic reactions that invoke a role for fast 'promoting' (or 'compressive') motions or vibrations that, in principle, can facilitate enzyme-catalysed reactions. With an emphasis on hydrogen-transfer reactions, experimental, theoretical and computational approaches that have lent evidence to this controversial hypothesis are discussed.
- Sam Hay
- & Nigel S. Scrutton
-
Article |
 Regio- and stereodivergent antibiotic oxidative carbocyclizations catalysed by Rieske oxygenase-like enzymes
Regio- and stereodivergent antibiotic oxidative carbocyclizations catalysed by Rieske oxygenase-like enzymes
Enzyme-mediated oxidative cyclizations in nature are a powerful demonstration of the utility of selective C–H activation. Here, Rieske oxygenase-like enzymes RedG and McpG are shown to mediate regio- and stereodivergent carbocyclization of undecylprodigiosin to streptorubin B and metacycloprodigiosin, respectively. Understanding these remarkably selective C–H activations could inspire the design of biomimetic catalysts with similar capabilities.
- Paulina K. Sydor
- , Sarah M. Barry
- & Gregory L. Challis
-
Article |
 Stereoselective C–C bond formation catalysed by engineered carboxymethylproline synthases
Stereoselective C–C bond formation catalysed by engineered carboxymethylproline synthases
The reaction of enols and enolates with electrophiles is used extensively in synthesis. Here, protein engineering — substituting amino acid residues in an enzyme active site — is used to produce biocatalysts for the control of enolate chemistry. The adapted enzymes enable stereoselective C–C bond formation yielding N-heterocycles in high diastereomeric excess by the reaction of trisubstituted-enolates.
- Refaat B. Hamed
- , J. Ruben Gomez-Castellanos
- & Christopher J. Schofield
-
News & Views |
 Diversity for self-assembly
Diversity for self-assembly
Self-assembly typically occurs through reversible interactions that slowly arrange building blocks into the most thermodynamically favoured structure. The involvement of enzymatic catalysis in the process has now enabled the rapid construction of a variety of low-defect architectures.
- Ehud Gazit
-
Article |
 Biocatalytic induction of supramolecular order
Biocatalytic induction of supramolecular order
Supramolecular gels show promise in diverse areas, including healthcare and energy technologies, owing to tunable properties that arise directly from the organization of their building blocks. Researchers have now been able to control this behaviour by combining enzymatic catalysis with molecular self-assembly. Although it seems counter-intuitive, gels that assembled faster showed fewer defects.
- Andrew R. Hirst
- , Sangita Roy
- & Rein V. Ulijn
-
News & Views |
 Sweet flexibility
Sweet flexibility
An enzyme that is unusually tolerant of a truly broad range of substrates can catalyse aldol-type chemistry on sugars in which the various hydroxyl groups are protected. The new methodology combines some of the most important advantages of enzyme and small-molecule catalysis.
- Benjamin G. Davis

 Ribozyme takes its vitamins
Ribozyme takes its vitamins