Abstract
The pattern recognition receptor TLR4 is well known as a crucial receptor during infection and inflammation. Several TLR4 antagonists have been reported to inhibit the function of TLR4. Both natural occurring antagonists, lipopolysaccharide (LPS) from Gram-negative bacteria as well as synthetic compounds based on the lipid A structure of LPS have been described as potent inhibitors of TLR4. Here, we have examined the characteristics of a natural TLR4 antagonist, isolated from Bartonella quintana bacterium by elucidating its chemical primary structure. We have found that this TLR4 antagonist is actually a lipooligosaccharide (LOS) instead of a LPS, and that it acts very effective, with a high inhibitory activity against triggering by the LPS-TLR4 system in the presence of a potent TLR4 agonist (E. coli LPS). Furthermore, we demonstrate that B. quintana LPS is not inactivated by polymyxin B, a classical cyclic cationic polypeptide antibiotic that bind the lipid A part of LPS, such as E. coli LPS. Using a murine LPS/D-galactosamine endotoxaemia model we showed that treatment with B. quintana LPS could improve the survival rate significantly. Since endogenous TLR4 ligands have been associated with several inflammatory- and immune-diseases, B. quintana LPS might be a novel therapeutic strategy for TLR4-driven pathologies.
Similar content being viewed by others
Introduction
The innate immune system is able to recognize multiple microbial components, including those of Gram-positive and Gram-negative bacteria, fungi and viruses. Recognition of Gram-negative bacteria mainly occurs via lipopolysaccharide (LPS), one of the major components of the outer membrane of these bacteria. The lipid A moiety of LPS interacts with a membrane receptor complex containing Toll-like receptor 4 (TLR4), MD-2, and CD14 and thereby induces proinflammatory cytokines, chemokines, and adhesion molecules. Together, these mediators may evoke the clinical signs of bacteria-induced sepsis1,2,3. Apart from microbial ligands, TLR4 is able to recognize endogenous ligands, such as breakdown products of extracellular matrix, alarmins, and intracellular proteins4,5,6,7,8. It has been suggested that these endogenous TLR4 ligands are important for the vicious loop during chronic inflammation9,10,11 and hence TLR4 is linked to the pathogenesis of several autoimmune diseases including rheumatoid arthritis, systemic lupus erythematosus, systemic sclerosis, Sjogren’s syndrome, psoriasis, multiple sclerosis, Atherosclerosis, and autoimmune diabetes12,13,14. The inhibition of TLR-4 activation has been investigated as potential anti-inflammatory therapy for many inflammatory diseases, including rheumatoid arthritis15.
Bartonella quintana is a louse-borne Gram-negative pathogen, which has been originally described during World War I as the causative microbe of trench fever, a disease associated with recurrent fever and headaches. B. quintana bacteria colonize the louse alimentary tract enabling a single louse to infect multiple humans16,17,18,19,20. After introduction into the human host, B. quintana can persist in the normally sterile bloodstream for weeks or month. This remarkable, prolonged persistence in the host bloodstream demonstrates the ability of B. quintana to avoid clearance by the host immune defense21. Furthermore, it has been observed that patients with B. quintana bacteremia do not show the classical sepsis syndrome. As an explanation for this phenomenon, overproduction of the anti-inflammatory cytokine interleukin-10 (IL-10) and an attenuated inflammatory cytokine profile during B. quintana bacteremia have been proposed22. We have previously described the anti-inflammatory effect of B. quintana LPS. The molecule blocks TLR4 activation and it has been shown that in several in vitro and in vivo models B. quintana LPS can be used as a potential therapeutic agent for the treatment of rheumatoid arthritis, ventilation-induced lung injury (VILI), atherosclerosis and other autoinflammatory diseases15,23,24,25. In the present study, we investigated the properties of B. quintana LPS in more details, in terms of induction of cytokines (pro- and anti-inflammatory), the potency to block TLR4, the kinetics of TLR4 antagonism and interaction with TLRs and other species of LPS.
Results
Bartonella quintana LPS does not induce production of pro- or anti-inflammatory cytokines
The first sets of experiments were designed to investigate whether exposure to B. quintana LPS results in the induction of pro- or anti-inflammatory cytokines by human PBMCs. As shown in Fig. 1, B. quintana LPS itself does not induce the production IL-1β, TNF-α, IL-6 or IL-8. In addition, exposure for 24 h with B. quintana LPS did not result in the production or release of IL-1Ra, or IL-10 by human primary PBMCs (data not shown). However, B. quintana LPS efficiently blocks production of IL-1β, TNF-α, IL-6, IL-8 or after stimulation of human PBMCs with E. coli LPS, indicating the potency of B. quintana LPS as TLR4 antagonist. In addition, we performed dose-response experiments to examine the IC50 of B. quintana LPS for the standard dose of 10 ml E. coli LPS. Figure 2A shows that already 20 ng of B. quintana LPS reduced the IL-6 production. At higher concentrations of B. quintana LPS, 5-fold or higher, a strong suppression of the IL-6 production was stated. Figure 2B demonstrated that B. quintana LPS revealed to have an IC50 of 37.04 ng/ml at a dose of 10 ng/ml ultra pure E. coli LPS. These data confirmed that LPS isolated of B. quintana is a very potent TLR4 antagonist at a low concentration23.
Human PBMCs were isolated from healthy subjects, using a standard protocol. PBMCs were pre-incubated with 100 ng/ml B. quintana LPS for 2 h, and thereafter 10 ng/ml purified E. coli LPS was added as indicated in the graph. Cytokines were determined after 24 h of culture by ELISA, IL-1β (A), TNF-α (B), IL-6 (C) and IL-8 (D). PBMCs of 6 healthy donors were examined. *P < 0.001, two-sided Mann-Whitney U test.
Human PBMCs were isolated from healthy subjects, using a standard protocol. PBMCs were pre-incubated with a dose-range of B. quintana LPS (10–1000 ng/ml) for 2 h. Thereafter, 10 ng/ml E. coli LPS was added and the PBMCs were cultured for another 24 h. IL-6 was determined by using ELISA (A). (B) Percentage inhibition was calculated using the IL-6 concentration of E. coli LPS exposure as 100%. PBMCs of 6 subjects were used in this experiment. IC50 was 37.04 ng/ml. *P < 0.001, two-sided Mann-Whitney U test.
Prolonged blocking of TLR4 by B. quintana LPS
In order to investigate the kinetics of the bindings capacity of B. quintana LPS to TLR4 and whether removing of the TLR4 inhibitor has an effect on blockade TLR4 function, we pre-incubated human PBMCs with B. quintana LPS for 1 hour. Two different approaches were investigated. The first approach was that B. quintana LPS was continuously present during the exposure to E. coli LPS and in the second approach we removed the B. quintana LPS by thorough washing (3 times). Thereafter the PBMCs were exposed to E. coli LPS and cells were incubated for additional 24 h, 48 h or 72 h hours. At each time point the cells were microscopically checked and the supernatant was collected to measure IL-1β, IL-6, IL-8, or TNF-α. Figure 3 shows that B. quintana LPS blocks the cytokine production by E. coli LPS at least for a period of 72 h. Cytokine production by human PBMCs is reduced for more than 90% over this exposure period, when the B. quintana LPS is present in the culture medium. The second approach in which B. quintana LPS was removed after 1 hour by repeated washing, identical effects on the neutralizing capacity of B. quintana LPS were seen (Fig. 4): Almost complete inhibition of the E. coli LPS-induced cytokine production after 72 h culture in the presence of 10 ng/ml of this classical TLR4 agonist. Thus, our data show that the blocking of TLR4 by B. quintana LPS is strong and stable for at least 72 hours.
Human PBMCs were isolated from healthy subjects, using a standard protocol. PBMCs were pre-incubated with 100 ng/ml or 1000 ng/ml B. quintana LPS for 2 h. Thereafter 10 ng/ml purified E. coli LPS was added as indicated in the graph. Cytokines were determined after 24 h, 48 h or 72 h of culture by standard ELISA for IL-1β (A), TNF-α (B), IL-6 (C) and IL-8 (D). PBMCs of 6 healthy donors were examined. *P < 0.001, two-sided Mann-Whitney U test.
Human PBMCs were isolated from healthy subjects, using a standard protocol. PBMCs were pre-incubated with 100 ng/ml or 1000 ng/ml B. quintana LPS for 2 h. Thereafter the PBMCs were extensively washed (3 times) to removed the non-bound B. quintana LPS. After the washing step, 10 ng/ml purified E. coli LPS was added. Cytokines were determined after 24 h, 48 h or 72 h of culture by standard ELISA for IL-1β (A), TNF-α (B), IL-6 (C) and IL-8 (D). PBMCs of 6 healthy donors were examined. *P < 0.001, two-sided Mann-Whitney U test.
Rapidity of binding of B. quintana LPS to TLR4
To explore the capacity of B. quintana LPS to bind the TLR4 over time, we examined the minimal pre-incubation time that allows B. quintana LPS to block TLR4 completely. Therefore we pre-incubated PBMCs with 100 ng/ml and 1000 ng/ml B. quintana LPS for 15, 30, 45 or 60 minutes and added 10 ng/ml E. coli LPS. After 24 h the IL-6 concentrations were determined in the supernatants. Figure 5A shows that 15 minutes’ pre-incubation is sufficient for 100 ng/ml of B. quintana LPS to block TLR4 receptor. The rapid binding of B. quintana LPS to TLR4 indicates that this TLR4 inhibitor is efficient.
Human PBMCs were isolated from healthy subjects, using a standard protocol. (A) PBMCs were pre-incubated with 100 ng/ml or 1000 ng/ml B. quintana LPS for different times before E. coli LPS was added to the culture medium. After 1 hour, 45, 30 and 15 minutes PBMC were exposed to 10 ng/ml E. coli LPS for 24 h. (B) B. quintana LPS was added together with E. coli LPS, thereafter the PBMCs were cultured for additional 24 h, 48 h or 72 h. C, B. quintana LPS was added in a range before (−2 h) and after (+2 h) the cells were exposed to 10 ng/ml E. coli LPS. IL-6 was determined by using ELISA. PBMCs of 4 subjects were used in this experiment. *P < 0.001, two-sided Mann-Whitney U test.
B. quintana LPS neutralizes TLR4 even in the presence of E. coli LPS
Since we noted that B. quintana LPS binds very rapidly to TLR4, we investigated the neutralizing capacity of B. quintana LPS when added together with E. coli LPS or even after addition of E. coli LPS to the culture medium. Figure 5B indicates that B. quintana LPS added together with E. coli LPS blocks the TLR4 receptor for at least 72 hours. IL-6 production due to 10 ng/ml E. coli LPS was completely suppressed by 10 times excess of B. quintana LPS. Remarkably, when B. quintana LPS was added 2 hours after the PBMC were exposed to E. coli LPS, we still noted a strong suppression of the IL-6 production. Figure 5C reveals that even a dose of 100 ng/ml B. quintana LPS (10 times excess) blocks TLR4 for 72 hours, in the presence of the TLR agonist.
Polymyxin B does not inactivate the antagonistic effect of B. quintana LPS
It is well known that polymyxin B binds to LPS from several Gram-negative microorganisms and neutralizes the activity. First, we compared B. quintana LPS with polymyxin B to analyze the difference in the neutralizing capacity. Figure 6A,B showed that B. quintana LPS is far more potent to inhibit E. coli LPS mediated TNF-α or IL-6 production by human PBMCs. A concentration of 100 ng/ml B. quintana LPS was equally potent as 10 μg/ml polymyxin B to block 1 or 10 ng/ml of E. coli LPS. Thereafter, we investigated whether polymyxin B was able to bind and inactivate B. quintana LPS. Figure 6C demonstrated that after pre-incubation of B. quintana LPS with 10 μg/ml polymyxin B for 2 hours, the inhibitory capacity of B. quintana LPS was still very high. As control, polymyxin B neutralized the E. coli LPS as expected.
Human PBMCs were isolated from healthy subjects, using a standard protocol. (A,B) PMBCs were exposed to a dose range of E. coli LPS (0.0001 to 10 ng/ml) in the presence or absence of B. quintana LPS (100 and 500 ng/ml) or polymixin (B) (3 and 10 μg/ml). TNF-α and IL-6 were determined with ELISA. (C) B. quintana LPS (100 ng/ml or 1000 ng/ml) was pre-incubated with 10 μg/ml polymyxin B for 2 h. Thereafter control medium, B. quintana LPS, polymyxin (B) B. quintana LPS + polymyxin B were added to the PBMCs for 2 h and thereafter the cells were washed 3 times with warm RPMI 1640 medium. After washing, RPMI 1640 medium or E. coli LPS (10 ng/ml) was added and the PBMCs were incubated for 24 h. IL-6 was determined by using ELISA. PBMCs of 4 subjects were used in this experiment. *P < 0.001, two-sided Mann-Whitney U test.
B. quintana LPS showed efficacy in E. coli LPS-induced murine model of endotoxaemia
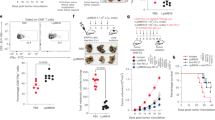
To explore whether B. quintana LPS can be used for in vivo studies to neutralize TLR4, we administered B. quintana LPS in an endotoxemia model. B. quintana LPS was injected 30 minutes before a sub-lethal dose of E. coli LPS was injected in combination with D-galactosamine. One single injection of B. quintana LPS revealed to be protective as can be seen in Fig. 7A,B. In contrast to the LPS/D-galactosamine group (30% survival after 10 days), B. quintana LPS administration had a significant higher survival rate (60%). As expected, injection of 100 μg B. quintana LPS alone had no detrimental effect on the survival of the mice.
C57/Bl6 mice were i.p. injected with either PBS (n = 20) or 100 μg B. quintana LPS (n = 20). After 30 minutes 10 mice of each group were injected i.p. with PBS and 10 mice were injected i.p. with E. coli LPS (1 μg) + D-galactosamine (14 mg). Survival was monitored for 10 days. (A) Survival rate. (B) Area under the curve. ***p < 0.01, one-way ANOVA test.
Structural analysis of B. quintana LPS
In order to elucidate the structure of B. quintana LPS we performed gas liquid chromatography and mass spectrometry (GLC-MS) and electrospray ionization Fourier transform ion cyclotron resonance (ESI FT-ICR) analysis. The LPS of B. quintana appears in the MS analysis with a molecular mass of 2438,448 Dalton. By examining fragmentation products of 30 V spectra it reveals that the sugar composition of B. quintana LPS contains 2 Kdo, 1 HexNAc and 2 HexN. The lipids were further characterized by GLC-M and the fatty acids 25-OH C26:0, 2 3-OH C16:0, 3-OH C12:0 and 3-OH C12:1 were detected. Figure 8 showed the predicted structure of B. quintana LPS and it reveals that the TLR4 antagonist is actually a lipooligosaccharide (LOS).
The primary sequence was depicted by Zähringer et al.57 for the equivalent molecule of B. henselae. Gas chromatographic analysis and mass spectrometry present differences of the B. quintana in comparison to the B. henselae LPS in the sugar composition and the acyl chains. The former contains a N-acetylated hexose in the outer core, while for the latter a hexose is described. Furthermore, one of the 3-OH C12 fatty acids is unsaturated in the LPS of B. quintana. For both substitutions the structures are not elucidated yet and the drawing shows only one of several possible positions.
To determine the aggregate structure of B. quintana LPS, small-angle X-ray scattering (SAXS) at the Hamburg synchrotron source PETRA was applied. For this, LPS at a concentration of 1 mg/50 μl was analyzed at two temperatures 20 and 40 °C (Fig. 9). The scattering patterns are indicative of a main maximum at d = 6.67 and 6.29 nm for 20 and 40 °C, respectively, and further reflections each at d/2, d/3, and d/5, which can be assigned to a multi-lamellar aggregate structure of the LPS dispersion.
Discussion
Here we described the in-vitro and in-vivo characteristics of the natural TLR4 antagonist B. quintana LPS. LPS of B. quintana appears to be a very potent, rapidly binding TLR4 blocker of a potent TLR4 agonist (E. coli LPS). In addition, the blockade of TLR4 is prolonged: at least 72 h after exposure to human PBMCs the effect persists. Since TLR4 activation is associated with many inflammatory and autoimmune diseases, B. quintana LPS might be considered a new therapeutic strategy for TLR4-driven pathology.
Bartonella quintana is an emerging Gram-negative pathogen, which may cause endocarditis, cerebral abscess and bacillary angiomatosis usually with the absence of septic shock in humans. Nowadays, the B. quintana infection can be found in homeless people, mainly due to body lice26. It has been reported in the past that LPS, isolated from B. quintana is able to induce proinflammatory cytokines when injected in rats27. This is disagreement with our results, in which highly purified B. quintana LPS showed no induction of cytokines, neither at the protein level nor at the level of gene transcription23. The main difference between our results and those previously reported by Matera et al., is the purity of B. quintana LPS. Due to contamination in the crude B. quintana extract, predominantly peptidoglycan-driven stimulation of cells through TLR2 ligations may occur. It revealed that Matera et al. used only a single isolation step to obtain B. quintana LPS (only the first step in our isolation and purification procedure of ultrapure B. quintana LPS). It is very likely that this crude preparation of B. quintana LPS activates human PBMCs in a TLR2-dependent pathway since peritoneal macrophages obtained from TLR2ko mice did not respond crude B. quintana LPS in vitro, in contrast to wild type mice (data not shown).
Here we described the structure of B. quintana LPS for the first time. It revealed that B. quintana LPS has 5 fatty acid tails, two of C12, two of C16 and one very long C26 (Fig. 8). It has been shown previously that LPS structures with 4 fatty acid chains are endotoxically inactive28. However, B. quintana LPS consists of 5 fatty acids and still is acts as a very potent TLR4 antagonist. This is in line with several other reports demonstrating that LPS originated from Gram-negative bacteria, such as Bradyrhizobium elkanii consists of 5 fatty acids tails and reveals to have antagonistic properties29,30. Studies using small angle X-ray scattering (SAXS) technology indicated that this particular LPS has a multilamellar structure. The data for the aggregate structure of LPS from B. quintana, a multilamellar organization, are characteristic for bioinactive structures of endotoxins similar as described by Brandenburg et al. and Schromm et al.31,32. In this kind of aggregate structure, the binding epitopes in LPS to the TLR4 receptor necessary for cell signaling are hidden, in contrast to the situation for bioactive LPS with its cubic aggregate structure31. However, since the cell activation is a membrane step and also a multilamellar LPS can incorporate into the immune cell membrane, cell receptors such as TLR4 may be blocked by them in this way inhibiting the cell signaling via bioactive LPS. A further observation in accordance to the chemical analysis described here should be mentioned: The periodicities in the range of 6.3 to 6.7 nm as shown in Fig. 9 are characteristic also for multi-lamellar structures of LPS from rough mutant Re and/or Rd from Salmonella minnesota, which in a previous report33 were found to result from the addition of divalent cations such as Mg2+ or at low water content. The final structure of the TLR4 antagonist revealed that the particular molecule is a lipooligosaccharide (LOS) and not a classical lipopolysaccharide (LPS).
Many TLR4 antagonists are based on LPS or lipid A structures obtained from non-pathogenic bacteria such as Rhodobacter capsulatus and Rhodobacter sphaeroides34. Compounds like E5531 (analogue of R. capsulatus lipid A) or Eritoran/E5564 (based on R. sphaeroides lipid A) were developed for the treatment of sepsis. In line with our results, Eritoran is significantly protective in animal models of sepsis35. In general, the TLR4 antagonists based on lipid A binds to MD-2 and thereby prevents binding of the agonist to the MD-2/TLR4 complex. This was interpreted to be due to the multilamellar aggregate structure of these antagonists which do not represent a disturbance of the membrane architecture at the site of the receptors, in contrast to the behavior of the non-lamellar aggregate structures of hexaacylated agonistic LPS36. Our data show that B. quintana LPS has a similar mode of action as E5564. Recently, potent low molecular inhibitors of TLR4 have been reported that interfere with the TLR4-MD2 complex formation37.
Polymyxin B is an antibiotic primarily used for resistant Gram-negative infections and it is derived from the bacterium Bacillus polymyxa. It has a bactericidal action against almost all Gram-negative bacilli and polymyxin binds to the cell membrane and alters its structure, making it more permeable, resulting in death of the microbe. Polymyxin B is well known for its LPS neutralizing capacity in vitro. This can be correlated with the observation, that polymyxin B converts the aggregate structure of agonistic LPS into a multilamellar form38. In the case of B. quintana LPS, its aggregate structure is already multilamellar, and is not changed furthermore by polymyxin B Furthermore, the fluidization observed when PMB interacts with hexaacylated endotoxins is absent32,38.
Here we demonstrated that B. quintana LPS binds very rapidly to the TLR4 complex, within 15 minutes the B. quintana LPS prevents activation of TLR4. Even 2 h after E. coli LPS was added to the cell cultures, B. quintana LPS was able to prevent cytokine production. This delayed antagonistic effect was only reported for one other natural TLR4 antagonist, isolated from the cyanobacterium Oscillatoria planktothrix FP139. It was demonstrated that the LPS from this cyanobacterium could block DC maturation and activation even 6 h after E. coli LPS was added. In line with our report, the cyanobacterium LPS was able to prevent LPS/D-galactosamine induced lethal shock. Although, the dose needed for protection was much higher (750 μg per mouse) than we showed in this current report (100 μg per mouse), indicating the potency of B. quintana LPS.
Activation of TLR4 has been associated with many inflammatory diseases and infectious complications40,41. Therefore, many efforts have been taken to develop or identity potent inhibitors of TLR4 for in-vivo applications. Apart from lipid A-derived structures or small molecules that interfere with MD-2/TLR4 formation42, antibodies have been developed. However, it seems that anti-TLR4 antibodies do not bind only TLR4, but via the Fc portion also to FcγRs. This dual action of these anti-TLR4 antibodies may be of importance to target inflammatory cells that express both receptors43.
Apart from infectious agents that can trigger TLR4 signaling, several endogenous TLR4 ligands have been described in the recent years. Many damage-associated products (DAMP) and inflammatory mediators have been linked to TLR4 for their pro-inflammatory behavior. Most of these TLR4 ligands are released after cells or tissues have been activated or damaged. A few examples of these endogenous TLR4 ligands are HMBG1, S100A7/8, fibronectin extra domain A and fetuin7,44,45,46,47. Potent inhibitors of TLR4 that interfere with both microbial TLR4 ligands as well as endogenous TLR4 ligands binding to TLR4 will have significant therapeutic value. Since the most TLR4 inhibitors are based on the disruption of the TLR4/MD-2 complex, which is very specific for lipid A-derived compounds, it remains to be explored whether TLR4 antagonist can be generated that block both classes of TLR4 ligands. Of high interest, B. quintana LPS reveals to inhibit both exogenous and endogenous TLR4 as previously reported15,48. Further investigation is warranted to elucidate the structure of B. quintana LPS to obtain insight into the mode of action and the possibility to synthesize this potent TLR4 antagonist.
Materials and Methods
The authors confirm that all experiments were performed in accordance with relevant guidelines and regulations. Written informed consents were obtained from all donors in accordance with the ethical principles set out in the declaration of Helsinki. The ethical review board of the Radboud University Medical Center, Nijmegen, The Netherlands, approved the study in which blood were used for healthy subjects (CMO2299 2010/104). The experimental protocols for murine studies were approved by the ethic committee for animal experiments (DEC) of the Radboud University Medical Center, Nijmegen, The Netherlands.
Reagents and microorganisms
LPS (E. coli serotype O55:B5) was purchased from Sigma Chemical Co and the Bartonella quintana CIP 103739 strain was kindly provided by Dr. Tanja Schulin and grown on sheep blood agar at 37 °C in a 5% CO2 atmosphere. B. quintana LPS was extracted by a two-step extraction method, which eliminates contamination with proteins. B. quintana LPS was extracted by hot phenol-water method as described previously23,49. Briefly, Bartonella quintana bacteria were scraped from blood agar plates, resuspended in PBS and heat-inactivated for 60 min in 56 °C. Thereafter, heat killed bacteria were wash twice with PBS and centrifuged for 10 min. at 16,262 × g. 2 grams of bacterial mass was used to isolate the LPS. Warm water (65 °C) was added to the pellet and the solution was vortexed for 10 minutes. Thereafter, the heated phenol (65 °C) was added and the solution was stirred for 2 hours at a temperature between 63–68 °C. Thereafter, solution was centrifuged 4,435 × g for 40 min at 4 °C. The aqueous phase was collected and transferred to a dialysis cassette (3.500 MWCO) and dialyzed against demi water in a 3L glass beaker in the cold room. The distilled water was changed after 30 minutes for the first time and then after 1 hour for 3–4 times. The LPS was dialyzed for two days at 4 °C, changing demi water 3 times a day. The dialyzed LPS was extracted and stored at −80 °C for until lyophilizing. For re-purification, 5mg Bartonella quintana LPS was added to 1 ml 0.2% TEA (Triethylamine)/0.5% Na-DOC (Natrium deoxycholate). Thereafter, 1 ml warm (60 °C) phenol:water (9:1 V/V) was added and the solution was vortexed for 5 min. After separation of the phases (5 minutes at 4 °C) the solution was centrifuged for 40 min. at 6,652 × g (4 °C). The water phase was collected and transferred to new sterile 15 ml tube. To the first phenol phase again 1 ml 0.2% TEA/0.5% Na-DOC was added and the previous steps were repeated. The second phenol phase was used to repeat the purification steps for the third time. The water phase of last 2 steps were combined with the first step. The LPS was dialyzed as described above, using the 3,500 MWCO cassette. To the dialyzed LPS drop-by-drop 1.5 ml of NaAc/EtOH (0.4 M in 100% EtOH) per each 0.5 ml of LPS was added and the solution was kept for 1 h on ice/water to let the LPS precipitate. Thereafter, the LPS was collected by centrifugation (30 min. at 16,262 × g) and washed twice with 1.5 ml cold EtOH followed by centrifugation (30 min. at 16,262 × g). Thereafter, LPS was dried on air, dissolved in PBS, aliquoted and stored by −20 °C. E. coli LPS from Sigma was also double purified, as described above.
Isolation of PBMC and stimulation of cytokine production
Peripheral blood mononuclear cells (PBMCs) were isolated healthy individuals (written informed consent was obtained from all subjects), as described earlier50,51. Briefly, PBMCs were isolated by density gradient centrifugation using Ficoll-Paque PLUS (GE Healthcare) and collecting the white interphase. Next, PBMCs were washed twice in cold PBS and concentrations were adjusted to 5 × 106 cells/ml in RPMI-1640 Dutch Modified culture medium (RPMI supplemented with 2 mM l-glutamine, 1 mM pyruvate; GIBCO Invitrogen, Carlsbad, CA, USA). PBMC (5 × 105), in a volume of 100 μl volume in round-bottomed 96-well plates (Greiner, Alphen a/d Rijn, The Netherlands), were incubated with either 100 μl of culture medium (negative control) or one of the following stimuli: B. quintana LPS and E. coli LPS (10 ng/ml).
Measurement of cytokine concentrations
Cytokines were determined by commercially available ELISA kits according to manufacturer’s instructions. Concentrations of human IL-1β, TNF-α, (R&D Systems, Inc., Minneapolis, MN, USA), IL-6 and IL-8 (Sanquin Reagents, Amsterdam, The Netherlands) were measured52,53.
Animals
C57Bl/6J mice were purchased from Charles River (Sulzfeld, Germany). For the experiments, 8–12 week old mice, weighing 20–25 g, were used. The animals were fed standard laboratory chow (Hope Farms, Woerden, The Netherlands) and housed under specific pathogen-free conditions. The experimental protocols were approved by the ethic committee for animal experiments (DEC) of the Radboud University Medical Center, Nijmegen, The Netherlands.
Experimental endotoxaemia model
The previously reported model of endotoxemia was used54. Briefly, 20 wild type mice were injected intraperitoneally (i.p.) with either PBS, or B. quintana LPS 100 μg/mouse. After 30 minutes 10 mice of each group were injected with PBS and 10 mice were injected i.p. E. coli LPS 1 μg per mouse (Escherichia coli LPS 055:B5 Sigma Chemical Co., St Louis, MI, USA) + D-galactosamine 14 mg per mouse. Survival of all 4 groups (PBS + PBS, B. quintana LPS + PBS, PBS + E. coli LPS/D-galactosamine and B. quintana LPS + E. coli LPS/D-galactosamine was assessed for 10 days.
Structural analysis of B. quintana LPS
The compositional analysis was done by using combined gas liquid chromatography and mass spectrometry (GLC-MS), as well as electrospray ionization mass spectrometry (ESI-MS). For the GLC-MS the B. quintana LPS was methanolyzed by 2 M HCL/CH3OH for 24 h at 85 °C and for the determination of the hexoses afterwards peracetylated or trimethylsilylated with N,O-bis(trimethylsilyl)trifluoroacetamide for the fatty acids, respectively. The resulting compounds were analyzed in a GLC on a Hewlett-Packard HP 5890 Series II chromatograph, equipped with a 30-m fused silica SPB-5 column (Supelco) using a temperature gradient of 150 °C (3 min) → 320 °C at 5 °C/min, and GLC-MS on a Hewlett-Packard HP 5989A instrument equipped with a 30-m HP-5MS column. Electrospray Ionization Fourier Transform Ion Cyclotron Resonance (ESI FT-ICR) MS was performed in using a hybrid Apex Qe FT-ICR MS instrument (Bruker Daltonics) in the negative ion mode, equipped with a 7 Tesla actively shielded magnet and an Apollo dual ion source55. Small-angle X-ray scattering (SAXS) measurements of LPS from Bartonella quintana were performed at the European Molecular Biology Laboratory outstation at the Hamburg synchrotron radiation facility (HASYLAB) using the double-focusing monochromator-mirror camera X33. Scattering patterns in the range of the scattering vector 0.01 < s < 1 nm−1 (s = 2 sinθ/λ, 2θ = scattering angle, λ = wavelength = 0.15 nm) were recorded at 20 and 40 °C with exposure times of 1 min using an image plate detector with online readout (MAR345; MarResearch, Norderstedt/Germany)56. The s-axis was calibrated with Ag-Behenate, which has a periodicity of 5.84 nm. We evaluated the diffraction by assigning the spacing ratios of the main scattering maxima to defined three-dimensional structures. For this study, the multi-lamellar structures were the most relevant, for which characteristic spacing’s at the periodicity d and further reflections at d/2, d/3 etc. are found.
Statistical analysis
The data are expressed as mean ± SEM. Differences between experimental groups were tested using the two-sided Mann-Whitney U test or one-way ANOVA performed on GraphPad Prism 6.0 software (GraphPad). P values of ≤0.05 were considered significant. The IC50 was calculated using non-linear concentration-response curve application within GraphPad Prism 6.0.
Additional Information
How to cite this article: Malgorzata-Miller, G. et al. Bartonella quintana lipopolysaccharide (LPS): structure and characteristics of a potent TLR4 antagonist for in-vitro and in-vivo applications. Sci. Rep. 6, 34221; doi: 10.1038/srep34221 (2016).
References
Miller, S. I., Ernst, R. K. & Bader, M. W. LPS, TLR4 and infectious disease diversity. Nat. Rev. Microbiol. 3, 36–46 (2005).
Fitzgerald, K. A., Rowe, D. C. & Golenbock, D. T. Endotoxin recognition and signal transduction by the TLR4/MD2-complex. Microbes Infect. 6, 1361–1367 (2004).
Kaisho, T. & Akira, S. Critical roles of Toll-like receptors in host defense. Crit. Rev. Immunol. 20, 393–405 (2000).
Pal, D. et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance Nat. Med. 18, 1279–1285 (2012).
Shi, B. et al. SNAPIN: an endogenous Toll-like receptor ligand in rheumatoid arthritis. Ann. Rheum. Dis. 71, 1411–1417 (2012).
Abdollahi-Roodsaz, S., Joosten, L. A., Koenders, M. I., van den Brand, B. T., van de Loo, F. A. & van den Berg, W. B. Local interleukin-1-driven joint pathology is dependent on toll-like receptor 4 activation. Am. J. Pathol. 175, 2004–2013 (2009).
Wu, H. et al. TLR4 activation mediates kidney ischemia/reperfusion injury. J. Clin. Invest. 117, 2847–2859 (2007).
Vogl, T. et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat. Med. 13, 1042–1049 (2007).
Goh, F. G., Piccinini, A. M., Krausgruber, T., Udalova, I. A. & Midwood, K. S. Transcriptional regulation of the endogenous danger signal tenascin-C: a novel autocrine loop in inflammation. J. Immunol. 184, 2655–2662 (2010).
Stifano, G. et al. Chronic Toll-like receptor 4 stimulation in skin induces inflammation, macrophage activation, transforming growth factor beta signature gene expression, and fibrosis. Arthritis Res. Ther. 16, R136 (2014).
Jia, L. et al. Hepatocyte Toll-like receptor 4 regulates obesity-induced inflammation and insulin resistance. Nat. Commun. 5, 3878–3885 (2014).
Liu, Y., Yin, H., Zhao, M. & Lu, Q. TLR2 and TLR4 in Autoimmune Diseases: Comprehensive Review. Clin. Rev. Allergy Immunol. 47, 136–147 (2014).
Den Dekker, W. K., Cheng, C., Pasterkamp, G. & Duckers, H. J. Toll like receptor 4 in atherosclerosis and plaque destabilization. Atherosclerosis 209, 314–320 (2010).
Kim, J. K. Fat uses a TOLL-road to connect inflammation and diabetes. Cell Metab 4, 417–419 (2006).
Abdollahi-Roodsaz, S. et al. Inhibition of Toll-like receptor 4 breaks the inflammatory loop in autoimmune destructive arthritis. Arthritis Rheum. 56, 2957–2967 (2007).
Foucault, C., Barrau, K., Brouqui, P. & Raoult, D. Bartonella quintana Bacteremia among Homeless People. Clinical Infect. Dis. 35, 684–689 (2002).
Foucault, C., Brouqui, P. & Raoult, D. Bartonella quintana characteristics and clinical management. Emerg. Infect. Dis. 12, 217–223 (2006).
Angelakis, E. & Raoult, D. Pathogenicity and treatment of Bartonella infections. Int J Antimicrob Agents 44, 16–25 (2014).
Liberto, M. C., Matera, G., Lamberti, A. G., Barreca, G. S., Quirino, A. & Foca, A. In vitro Bartonella quintana infection modulates the programmed cell death and inflammatory reaction of endothelial cells. Diagnostic Microbiol. Infect. Dis. 45, 107–115 (2003).
Matera, G. et al. The Janus face of Bartonella quintana recognition by Toll-like receptors (TLRs): a review. Eur. Cytokine Netw. 19, 113–118 (2008).
Pulliainen, A. T. & Dehio, C. Persistence of Bartonella spp. stealth pathogens: from subclinical infections to vasoproliferative tumor formation. FEMS Microbiology Reviews 36, 563–599 (2012).
Capo, C., Amirayan-Chevillard, N., Brouqui, P., Raoult, D. & Mege, J. L. Bartonella quintana bacteremia and overproduction of interleukin-10: model of bacterial persistence in homeless people. J. Infect. Dis. 187, 837–844 (2003).
Popa, C. et al. Bartonella quintana lipopolysaccharide is a natural antagonist of Toll-like receptor 4. Infect. Immun. 75, 4831–4837 (2007).
Den Dekker, W. K. et al. Mast cells induce vascular smooth muscle cell apoptosis via a toll-like receptor 4 activation pathway. Arterioscler. Thromb. Vasc. Biol. 32, 1960–1969 (2012).
Vaneker, M. et al. Low-tidal-volume mechanical ventilation induces a toll-like receptor 4-dependent inflammatory response in healthy mice. Anesthesiology 109, 465–472 (2008).
Drali, R. et al. Bartonella quintana in body lice from scalp hair of homeless persons, France. Emerg. Infect. Dis. 20, 907–908 (2014).
Matera, G. et al. Bartonella quintana lipopolysaccharide effects on leukocytes, CXC chemokines and apoptosis: a study on the human whole blood and a rat model. Int. Immunopharmacol. 3, 853–864 (2003).
Mueller, M., Brandenburg, K., Dedrick, R., Schromm, A. B. & Seydel, U. Phospholipids inhibit lipopolysaccharide (LPS)-induced cell activation: a role for LPS-binding protein. J. Immunol. 174, 1091–1096 (2005).
van den Plas, M. L. et al. Rhizobium sin-1 lipopolysaccharide (LPS) prevents enteric LPS-induced cytokine production. J. Biol. Chem. 277, 41811–41816 (2002).
Komaniecka, I., Choma, A., Lindner, B. & Holst, O. The structure of a novel neutral lipid A from the lipopolysaccharide of Bradyrhizobium elkanii containing three mannose units in the backbone. Chemistry 16, 2922–2929 (2010).
Brandenburg, K., Andrä, J., Müller, M., Koch, M. H. & Garidel, P. Physicochemical properties of bacterial glycopolymers in relation to bioactivity. Carbohydr. Res. 338, 2477–2489 (2003).
Schromm, A. B. et al. Physicochemical and biological analysis of synthetic bacterial lipopeptides: validity of the concept of endotoxic conformation. J. Biol. Chem. 282, 11030–11037 (2007).
Seydel, U., Koch, M. H. J. & Brandenburg, K. Structural polymorphisms of rough mutant lipopolysaccharides Rd to Ra from Salmonella minnesota. J Struct Biol. 110, 232–243 (1993).
Peri, F. & Piazza, M. Therapeutic targeting of innate immunity with Toll-like receptor 4 (TLR4) antagonists. Biotechnol. Adv. 30, 251–260 (2012).
Mullarkey, M., Rose, J., Bristol, J., Kawata, T., Kimura, A. & Kobayashi, S. Inhibition of endotoxin response by e5564, a novel Toll-like receptor 4 directed endotoxin antagonist. J. Pharmacol. Exp. Ther. 304, 1093–1102 (2003).
Brandenburg, K. & Wiese, A. Endotoxins:Relationship between structure, function, and activity. Curr. Top. Medicin. Chem. 4, 1127–1146 (2004).
Švajger, U. et al. Novel toll-like receptor 4 (TLR4) antagonists identified by structure- and ligand-based virtual screening. Eur. J. Med. Chem. 70, 393–399 (2013).
Brandenburg, K., David, A., Howe, J., Koch, M. H., Andrä, J. & Garidel, P. Temperature dependence of the binding of endotoxins to the polycationic peptides polymyxin B and its nonapeptide. Biophys. J. 88, 1845–18458 (2005).
Macagno, A. et al. A cyanobacterial LPS antagonist prevents endotoxin shock and blocks sustained TLR4 stimulation required for cytokine expression. J. Exp. Med. 203, 1481–1492 (2006).
Belcher, J. D. et al. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood 123, 377–390 (2014).
Zhang, X. et al. Toll-like receptor 4 plays a central role in cardiac dysfunction during trauma hemorrhage shock. Shock 42, 31–37 (2014).
Shirey, K. A. et al. The TLR4 antagonist Eritoran protects mice from lethal influenza infection. Nature 497, 498–502 (2013).
Shang, L. et al. Selective Antibody Intervention of Toll-like Receptor 4 Activation through Fc γ Receptor Tethering. J. Biol. Chem. 289, 15309–15318 (2014).
Andersson, U. & Tracey, K. J. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu. Rev. Immunol. 29, 139–162 (2011).
Ehrchen, J. M., Sunderkötter, C., Foell, D., Vogl, T. & Roth, J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection,autoimmunity, and cancer. J. Leukoc. Biol. 86, 557–566 (2009).
Schelbergen, R. F. et al. Alarmins S100A8 and S100A9 elicit a catabolic effect in human osteoarthritic chondrocytes that is dependent on Toll-like receptor 4. Arthritis Rheum. 64, 1477–1487 (2012).
Bhattacharyya, S. et al. Fibronectin EDA promotes chronic cutaneous fibrosis through Toll-like receptor signaling. Sci Transl Med. 6, 232ra50 (2014).
Abdollahi-Roodsaz, S. et al. Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J. Clin. Invest. 118, 205–216 (2008).
Morrison, D. C. & Leive, L. Isolation and characterization of two fractions of lipopolysaccharide from E. coli 0111:B4. J. Biol. Chem. 250, 2911–2919 (1975).
Mylona, E. E. et al. Enhanced interleukin-1β production of PBMCs from patients with gout after stimulation with Toll-like receptor-2 ligands and urate crystals. Arthritis Res. Ther. 14, R158 (2012).
Oosting, M. et al. Role of interleukin-23 (IL-23) receptor signaling for IL-17 responses in human Lyme disease. Infect. Immun. 79, 4681–4687 (2011).
Buffen, K. et al. Autophagy modulates Borrelia burgdorferi-induced production of interleukin-1β (IL-1β). J. Biol. Chem. 288, 8658–8666 (2013).
Kleinnijenhuis, J. et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 109, 17537–17542 (2012).
Weber, M. A. et al. Endogenous leukemia inhibitory factor attenuates endotoxin response. Lab. Invest. 85, 276–284 (2005).
Zehethofer, N. et al. Lipid Analysis of Airway Epithelial Cells for Studying Respiratory Diseases. Chromatographia 78, 403–413 (2015).
Roessle, M. et al. Upgrade of the small-angle X-ray scattering beamline at the European Molecular Biology Laboratory, Hamburg. J. Appl. Crystallogr. 40, 190–194 (2007).
Zähringer, U. et al. Structure and biological activity of the short-chain lipopolysaccharide from Bartonella henselae ATCC 49882T. J Biol Chem. 279, 21046–21054 (2004).
Acknowledgements
The authors would like to thank Dr. Patrick Sturm from the department of Microbiology, Radboud University Medical Center for the advice for large-scale culture of B. quintana. Helga Toenhake-Dijkstra and Heidi Lemmers are acknowledged for culturing of B. quintana and isolation of LPS of B. quintana. This study was supported by a NGI Pre-Seed grant (93610003) to LAB-J. MGN is supported by a Vici grant of the Netherlands Organization for Scientific Research and an ERC Consolidator Grant (nr. 310372).
Author information
Authors and Affiliations
Contributions
G.M.-M. and L.H. performed the experiments and prepared figures. G.M.-M., M.G.N. and L.A.B.J. wrote the main manuscript text. K.B. and J.W.M.v.d.M. reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
This study was supported by a grant of ZonMW (Pre-Seed Grant 93610003). The authors declare that J.W.M.v.d.M., M.G.N. and L.A.B.J hold a patent on Bartonella LPS (PCT/EP 2006/009528 Novel antagonist of the Toll-Like Receptor 4).
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Malgorzata-Miller, G., Heinbockel, L., Brandenburg, K. et al. Bartonella quintana lipopolysaccharide (LPS): structure and characteristics of a potent TLR4 antagonist for in-vitro and in-vivo applications. Sci Rep 6, 34221 (2016). https://doi.org/10.1038/srep34221
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep34221
This article is cited by
-
A systematic review on antibiotic therapy of cutaneous bacillary angiomatosis not related to major immunocompromising conditions: from pathogenesis to treatment
BMC Infectious Diseases (2024)
-
Toll-like receptor 4 (TLR4) antagonists as potential therapeutics for intestinal inflammation
Indian Journal of Gastroenterology (2021)
-
Whole genomic sequencing and sex-dependent abundance estimation of Cardinium sp., a common and hyperabundant bacterial endosymbiont of the American house dust mite, Dermatophagoides farinae
Experimental and Applied Acarology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.