Abstract
New diagnostic methods have provided a promising solution for rapid and reliable detection of drug-resistant TB strains. The aim of this study was to evaluate the performance of the MeltPro TB assay in identifying multidrug-resistant (MDR-) and extensively drug-resistant tuberculosis (XDR-TB) patients from sputum samples. The MeltPro TB assay was evaluated using sputum samples from 2057 smear-positive TB patients. Phenotypic Mycobacterial Growth Indicator Tube (MGIT) 960 drug susceptibility testing served as a reference standard. The sensitivity of the MeltPro TB assay was 94.2% for detecting resistance to rifampicin and 84.9% for detecting resistance to isoniazid. For second-line drugs, the assay showed a sensitivity of 83.3% for ofloxacin resistance, 75.0% for amikacin resistance, and 63.5% for kanamycin resistance. However, there was a significant difference for detecting kanamycin resistance between the two pilot sites in sensitivity, which was 53.2% in Guangdong and 81.5% in Shandong (P = 0.015). Overall, the MeltPro TB assay demonstrated good performance for the detection of MDR- and XDR-TB, with a sensitivity of 86.7% and 71.4%, respectively. The MeltPro TB assay is an excellent alternative for the detection of MDR- and XDR-TB cases in China, with high accuracy, short testing turn-around time, and low unit price compared with other tests.
Similar content being viewed by others
Introduction
Despite encouraging progress in tuberculosis (TB) control, TB remains a leading cause of morbidity and mortality worldwide1,2. The World Health Organization (WHO) estimated that there were 9 million incident TB cases and 1.5 million deaths from TB in 20133. The emergence of drug-resistant TB—especially multidrug-resistant TB (MDR-TB, defined as resistance to at least isoniazid and rifampicin) and extensively drug-resistant TB (XDR-TB, defined as MDR-TB plus resistance to any fluoroquinolone and kanamycin, amikacin, or capreomycin)—is considered the greatest obstacle to global TB control due to difficulties in diagnosis and treatment4,5,6. Globally, in 2013, an estimated 480,000 and 43,200 people developed MDR-TB and XDR-TB, respectively. However, only 136,000 MDR-TB cases (28%) were detected and notified, and the situation for detecting XDR-TB was even more unsatisfactory because of the lack of capability to detect susceptibility against second-line drugs in many TB high-burden countries3. Hence, there is an urgent need to ensure that all drug-resistant TB suspects undergo testing for susceptibility to anti-TB drugs to initiate effective treatment regimens and appropriate measures of infection control2,7.
Because of the slow growth rate of Mycobacterium tuberculosis (MTB), conventional phenotypic drug susceptibility testing (DST) typically takes 1 to 3 months to determine the drug resistance profiles of MTB isolates8,9,10. More importantly, all culture-based conventional methods require a biosafety category 3 laboratory facility and extensive training of personnel, requirements that are largely unattainable in developing countries10.
In recent years, several commercial molecular tests have been developed to determine the drug resistance of MTB isolates based on the detection of specific genetic mutations conferring resistance2,11,12,13,14. Of these rapid tests, the GenoType MTBDR (Hain Lifescience, Germany) and GeneXpert (Cepheid, USA) have been endorsed by WHO for the detection of rifampicin (RIF) and/or isoniazid (INH) resistance8. Although most available molecular diagnostics detect resistance to RIF and INH, the choice of molecular assays to detect resistance to second-line drugs is far more limited, except for GenoType MTBDRsl from Hain Lifescience7. On the basis of published reports, WHO decided not to recommend this assay as a replacement for conventional DST to rule out resistance in routine clinical practice15. The shortage of DST technologies for second-line drugs highlights the need for the development and evaluation of new molecular tools to improve diagnosis of MDR- and XDR-TB.
The MeltPro TB assay, developed by Zeesan Biotecheh (Xiamen, China), is an innovative molecular test for detection of resistance to the main first-line and second-line anti-TB drugs16. Unlike commercially available molecular tests, this technology is based on melting curve analysis with dually labeled probes, which retrieves the melting temperature (Tm) shift from the wide-type into the genetic mutation of MTB. The intrinsic feature of this assay makes it possible to cover two fragments (more than 20 continuous nucleotides per fragment) associated with drug resistance in one assay; it is also easy to perform without cumbersome hybridization16,17. The MeltPro TB assays for detecting RIF, INH and fluoroquinolone resistance have been officially approved by the China Food and Drug Administration (CFDA), marking them possible for clinical practice. Several previous studies found that the sensitivity and specificity of MeltPro were 93.4% and 97.4%, respectively, for RIF resistance, and 90.8% and 96.4%, respectively, for INH resistance using the proportion method on the solid medium as a reference standard16,18, suggesting that this assay could be used as an alternative to phenotypic DST. However, these studies evaluated the MeltPro TB assay on cultured isolates, and assay performance for detecting resistance to second-line drugs was not evaluated. Thus, there is a need to validate assay performance in settings with a high prevalence to evaluate the feasibility of using the test for routine detection of XDR-TB cases from clinical samples.
In this paper, we report on a multicenter study to evaluate use of the MeltPro TB assay on sputum samples for detection of resistance to first-line and second-line TB drugs. The results provide insight on the potential to scale up this new technology in China.
Results
Patient enrollment
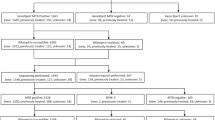
A total of 2057 smear-positive patients were enrolled in this evaluation. One specimen was collected from each patient and digested with NALC-NaOH for liquid DST and MeltPro TB assay. Figure 1 depicts how samples were processed and achieved. Of the specimens for liquid DST, 111 (5.4%) were negative in culture; 51 (2.5%) were contaminated; 137 (6.7%) were identified as nontuberculous mycobacteria by MPB64 monoclonal antibody assay; and the DST results for 28 (1.4%) specimens were invalid. For the MeltPro TB assay, 196 (9.5%) specimens were excluded from the study due to nontuberculous mycobacteria and invalid results. Overall, 1541 specimens were used for evaluating the performance of the MeltPro TB assay (Fig. 1).
Performance of MeltPro TB assay for RIF and INH resistance
We first evaluated the performance of the MeltPro TB assay versus liquid DST for detection of RIF and INH resistance. As shown in Table 1, among 278 RIF-resistant TB patients diagnosed by DST, 262 cases were identified by MeltPro TB assay, with a sensitivity of 94.2% (see Table 1 for confidence intervals). In addition, 1231 out of 1263 RIF-susceptible TB patients diagnosed by DST were confirmed by MeltPro TB assay, indicating a specificity of 97.5%. For INH resistance, the sensitivity and specificity of the MeltPro TB assay were 84.9% and 98.0%, respectively (Table 2). We also calculated MeltPro TB assay performance for MDR-TB detection measured against liquid DST. Overall, the sensitivity for MDR-TB was 86.7%, and the specificity was 97.7% (Table 3).
Performance of MeltPro TB assay for detecting resistance to second-line drugs
In comparison with conventional liquid DST as the gold standard, the MeltPro TB assay showed a sensitivity of 83.3% for OFLX, 75.0% for AMK, and 63.5% for KAN (Tables 4, 5, 6). The specificity for detecting resistance to all three drugs was greater than 98%: 98.1% for OFLX, 98.7% for AMK, and 99.2% for KAN. For OFLX and AMK, sensitivity was similar in both Shandong and Guangzhou. For KAN, however, there was a significant difference between the two pilot sites in sensitivity, which was 53.2% in Guangdong and 81.5% in Shandong (P = 0.015).
In view of the significant different performance of MeltPro for detecting KAN resistance between Shandong and Guangdong, the gene mutations of 74 phenotypically KAN-resistant strains were analyzed by DNA sequencing. As shown in Table 7, the mutation located in 1401 A→G of the rrs region was identified as the most frequent mutation conferring KAN resistance in both Shangdong (20/27, 74.1%) and Guangdong (19/47, 40.4%). In contrast, we also observed that there were 5 (18.5%) and 21 (44.7%) KAN-resistant isolates harboring no mutation in Shangdong and Guangdong, respectively. Statistical analysis revealed that there was a significantly higher proportion of KAN-resistant isolates with genetic mutations in Shandong in comparison with that in Guangdong (P = 0.023).
We also analyzed the sensitivity and specificity of the MeltPro TB assay for diagnosing XDR-TB cases. Overall, the accuracy of the MeltPro TB assay for detecting XDR-TB was 98.8%, with a sensitivity and specificity of 71.4% and 99.6%, respectively (Table 8).
Discussion
We performed the first multicenter study to access the diagnostic accuracy of the MeltPro TB assay for detection of MDR- and XDR-TB cases from clinical sputum samples. The assay demonstrated satisfactory sensitivity and specificity for drug-resistant M. tuberculosis among patients at hospitals with high burden of drug-resistant TB, especially for RIF and INH.
Several commercial diagnostic tools for detection of drug-resistant TB have been evaluated in laboratories of various types in China. These tools have included Genotype MTBDR from Hain, Genechip from CapitalBio, and GeneXpert from Cephid8,19,20. The sensitivities of these molecular assays for detection of RIF resistance have varied between 87.10% for GeneXpert20 and 88.3% for Genotype MTBDR19—lower than the sensitivity of 94.2% in this study. Several factors may be responsible for the difference. First, the detection of heteroresistance by the MeltPro assay is the major contributor to the increased sensitivity for detection of RIF resistance. Because of the inherent limitations of the interpretation system for Genechip, it is difficult to detect less than 50% RIF heteroresistance. For GeneXpert, the presence of susceptible bacteria would result in the occurrence of amplification curves, thereby resulting in missed detection of RIF heteroresistance. A prior study has demonstrated that a high melting curve assay can detect the presence of RIF resistance mutations down to a concentration of 5% mutant DNA21. Hence, given the high prevalence of heteroresistance in China22,23, the better capability of MeltPro to detect mixed infection may help laboratory staff identify more RIF-heteroresistant TB cases. Second, the different phenotypic DST methods used may be another explanation for the difference.
The sensitivity of MeltPro for INH resistance was also higher than that for the other evaluated methods. A recent molecular epidemiological study from China has demonstrated that the combination mutations in katG gene and the promoter of inhA gene can only identify about 75% of INH-resistant isolates, while the nucleotide substitutions in the intergenic region of oxyR-ahpC rather than other mutations confers 5.1% of INH resistance in the MDR population of China24. The inclusion of the ahpC promoter region therefore increased the test sensitivity (84.9%) of MeltPro to detect the INH resistance when compared with 80.34% for Genechip and 80.2% for Genotype MTBDR8,19.
In the current study, the MeltPro assay reliably detected OFLX resistance, with a sensitivity of 83.3% and a specificity of 98.1%. Unlike the situation with tests for RIF and INH resistance, the diagnostic accuracy of molecular tools shows significant heterogeneity for detection of fluoroquinolone (FQ) resistance across studies, varying from 68% to 92%15. On one hand, the frequencies of mutations conferring FQ resistance differ from one geographic region to another. For example, one study found that 83% of FQ-resistant TB isolates from Russia harbored gyrA mutations25, whereas this percentage in Taiwan was only 50%26. Hence, variation in the molecular characteristics of FQ-resistant isolates from region to region may be the primary reason for differences in diagnostic accuracy. On the other hand, heteroresistance is considered to be an important mechanism for the emergence of resistance, and a high rate of heteroresistance is associated with settings with a high TB prevalence22. In a recent study, Zhang and colleagues found FQ heteroresistance in 23% of TB isolates in China22, which is a significantly higher percentage than has been observed in South Korea and the United States, with a proportion of 9%21,22. Hence, in light of the poor sensitivity of molecular methods for detecting heteroresistance in comparison with phenotypic DST methods27, the relatively common occurrence of FQ heteroresistance may be attributed to variable performance of molecular methods for identifying FQ resistance from clinical samples.
In regard to second-line injectable drugs, the MeltPro assay showed moderate test sensitivity and high specificity for the detection of AMK resistance. By contrast, its sensitivity for predicting KAN resistance was unsatisfactory. The KAN-resistant strains isolated from Shandong are more likely to harbor genetic mutations located in the rrs gene and eis promoter than those from Guanzhou, resulting in higher sensitivity for the detection of KAN resistance. Our findings were in line with previous observations of significant variability in the sensitivity for the detection of KAN resistance across studies, ranging from 25.0% to 100.0%15. The different distribution of mutations with geographic origin provides a potential explanation for this heterogeneity. In line with our findings, several previous reports have demonstrated that the frequencies of mutants conferring KAN resistance show significant diversity among different regions of China, ranging from 54% in Chongqing to 100% in Hunan28,29. Hence, our findings suggest that the MeltPro assay can be used as an alternative for detecting OFLX and AMK resistance in China, although its feasibility for detecting KAN resistance is questionable. This indicates an urgent need for further studies in different settings of China.
According to the 12thnational five-year plan drawn up by the government of China, rapid drug susceptibility testing tools will be adopted overall in prefectural and municipal TB laboratories by 2015. To provide more detailed evidence for laboratory technicians, this paper compares the four available rapid DST methods (Table 9). First, based on our evaluation, the MeltPro assay shows the favorable performance for detecting RIF and INH resistance. Second, in regard to the simplicity of operation, GeneXpert is no doubt the most automatic platform for rapid identification of RIF resistance. Because of the application of automated nucleic acid extraction devices and an in-tube detection system, the MeltPro is more convenient than Genechip and GenoType MTBDR and offers a shorter turn-around time to generate diagnostic results. Third, unlike the three other available assays, the MeltPro uses a conventional real-time PCR platform rather than any special equipment to perform clinical analysis, which is more suitable for TB laboratories in poor regions. Finally, the MeltPro assay provides the lowest reagent price for detecting RIF and INH resistance—half that of GeneXpert. Hence, the MeltPro assay is a cost-effective method for MDR- and XDR-TB diagnosis, compared to both conventional DST methods and other commercial molecular kits available in China. It has important potential for improving diagnosis and control of drug-resistant TB.
There were several obvious limitations in this study. First, several literatures have revealed that liquid DST is prone to miss some low-level resistant MTB when compared with conventional solid DST method30. Hence, the gold standard used in this study may influence the analytical sensitivity and specificity of MeltPro. Second, whole-genome sequencing (WGS) has been considered as a useful tool for validating mutations and confirming molecular resistance compared to phenotypic DST7,31. However, due to the high price of WGS and the large sample size, WGS was not performed in the present study. Nevertheless, our evaluation provides important evidence for further implementation of MeltPro in the diagnosis algorithm of China.
In conclusion, our data demonstrate that the MeltPro assay is an excellent method for the detection of MDR- and XDR-TB cases in China. It outperforms several commercial assays in that it provides satisfactory accuracy, short testing turn-around time, and low unit price. Because we found significant heterogeneity for the detection of KAN resistance across regions, an effective evaluation of MeltPro needs to be conducted prior to scaling this assay for detecting KAN resistance in different regions of China. Further evaluation will also be needed to investigate the impact of the MeltPro assay on patient and TB program outcomes. Wide spread use of the assay may potentially lead to faster initiation of appropriate treatment for drug-resistant TB patients as well as reduced transmission of drug-resistant TB in the community.
Methods
Study sites and population
This study was approved by PATH and the Ethics Committee of Chinese Center for Disease Control and Prevention. The methods used in this study were in accordance with the approved guidelines. All patients enrolled in this study provided written informed consent for the samples collected for the research study protocol.
Between January 1, 2014, and February 28, 2015, the performance of MeltPro TB assay was evaluated in two TB-specialized hospitals in China: Guangzhou Chest Hospital and Shandong Chest Hospital. All smear-positive TB patients seeking health care in these two hospitals were enrolled consecutively in the study, irrespective of co-morbidities or HIV-status. We collected one sputum specimen of more than 2 mL from each patient for further smear microscopy, liquid culture, and MeltPro TB assay.
Smear and phenotypic DST
Direct smear was performed using light-emitting diode fluorescence microscopy for acid fast bacilli (AFB). Smears were graded according to national guidelines established by China Center for Disease Control and Prevention, which starts with negative to scanty to 4+8. A smear-positive specimen was digested by using the N-acetyl-L-cysteine (NALC)-NaOH method for 15 minutes, and then neutralized with sterile phosphate buffer (PBS, pH = 7.0). After centrifugation at 3,000 × g for 15 minutes, the pellet was resuspended in 2 mL PBS buffer. A 0.5 mL proportion of the decontaminated specimens was cultured on Mycobacterial Growth Indicator Tubes (MGIT, Becton Dickinson, USA). Positive cultures were confirmed as mycobacteria with Ziehl-Neelsen staining. Further species identification was performed using a commercial MPB64 monoclonal antibody assay (Genesis, Hangzhou, China). Indirect drug susceptibility of the culture-positive isolates identified as MTB was detected by the Bactec MGIT 960 automated system according to the manufacturer’s instructions. The critical concentrations were 1.0 μg/mL for rifampicin (RIF), 0.1 μg/ml for isoniazid (INH), 2 μg/mL for ofloxacin(OFLX), 0.5 μg/mL for amikacin (AMK), and 1.0 μg/mL for kanamycin (KAN)27,32,33. The clinical TB laboratories enrolled in this study have passed the proficiency testing for conventional DST organized by the National TB Reference Laboratory of the China CDC.
MeltPro TB assay
MeltPro TB assay testing was conducted following the manufacturer’s instructions. Briefly, the crude DNA was extracted from a 1.0 mL aliquot of the decontaminated specimens with an automatic DNA extraction machine (Zeesan Biotecheh, Xiamen, China) using the paramagnetic particle method. Five microliters of the genomic DNA was applied to the amplification in each tube. The PCR mixture was performed in the LightCycler 480 system (Roche Applied Science, Indianapolis, USA) according to the following protocol: 2 min of decontamination at 50 °C using uracil-N-glycosylase; 10 min of denaturation at 95 °C; a 10 cycles touchdown program containing 10 s at 95 °C, 15 s at 71 °C (−1 °C/cycle), and 15 s at 78 °C; and 45 cycles of 10 s at 95 °C, 15 s at 61 °C, and 15 s at 78 °C. Melting curve analysis was started with 2 min of denaturation at 95 °C, 2 min of hybridization at 40 °C, and a stepwise increasing temperature from 40 °C to 85 °C at 1 °C/step with a 5-s stop between each step. The fluorescent signal intensity was collected at FAM and TET channels. Tm calling analysis was performed by identifying the peaks of the melting curves. An invalid result was defined as the strain with invalid result of any drug susceptibility.
DNA sequencing
The crude genomic DNA was extracted from freshly cultured bacteria following the method described previously34. The genomic DNA was used as template to carry out PCR amplification. The fragments of genes conferring KAN resistance, including rrs and eis promoter, were amplified, and then sent to Qingke Company for sequencing service. DNA sequences were aligned with the homologous sequences of M. tuberculosis H37Rv strain (http://www.ncbi.nlm.nih.gov/BLAST).
Data analysis
Conventional liquid DST was used as the reference standard to calculate the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the MeltPro TB assay. All the data were entered into SPSS15.0 software as a database (SPSS Inc., USA). A chi-square test was used for statistical analysis. If the P value was less than 0.05, the difference was judged as significant.
Additional Information
How to cite this article: Pang, Y. et al. Rapid diagnosis of MDR and XDR tuberculosis with the MeltPro TB assay in China. Sci. Rep. 6, 25330; doi: 10.1038/srep25330 (2016).
References
Zumla, A. et al. Drug-resistant tuberculosis–current dilemmas, unanswered questions, challenges, and priority needs. J Infect Dis. 205, Suppl 2, S228–240 (2012).
Pai, M. & Schito, M. Tuberculosis diagnostics in 2015: landscape, priorities, needs, and prospects. J Infect Dis 211, Suppl 2, S21–28 (2015).
World Health Oragnization. Global tuberculosis report 2014. World Health Organization, Geneva, Switherland. WHO/HTM/TB/2014.08 (2014).
Wright, A. et al. Epidemiology of antituberculosis drug resistance 2002–07: an updated analysis of the Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Lancet 373, 1861–1873 (2009).
Van Rie, A. & Enarson, D. XDR tuberculosis: an indicator of public-health negligence. Lancet 368, 1554–1556 (2006).
Nachega, J. B. & Chaisson, R. E. Tuberculosis drug resistance: a global threat. Clin Infect Dis 36, S24–30 (2003).
Tagliani, E. et al. Diagnostic Performance of the New Version (v2.0) of GenoType MTBDRsl Assay for Detection of Resistance to Fluoroquinolones and Second-Line Injectable Drugs: a Multicenter Study. J Clin Microbiol 53, 2961–2969 (2015).
Pang, Y. et al. Multicenter evaluation of genechip for detection of multidrug-resistant Mycobacterium tuberculosis. J Clin Microbiol 51, 1707–1713 (2013).
Hoek, K. G., Van Rie, A., van Helden, P. D., Warren, R. M. & Victor, T. C. Detecting drug-resistant tuberculosis: the importance of rapid testing. Mol Diagn Ther 15, 189–194 (2011).
Drobniewski, F., Nikolayevskyy, V., Balabanova, Y., Bang, D. & Papaventsis, D. Diagnosis of tuberculosis and drug resistance: what can new tools bring us? Int J Tuberc Lung Dis 16, 860–870 (2012).
O’Grady, J. et al. New and improved diagnostics for detection of drug-resistant pulmonary tuberculosis. Curr Opin Pulm Med 17, 134–141, 10.1097/MCP.0b013e3283452346 (2011).
Ling, D. I., Zwerling, A. A. & Pai, M. GenoType MTBDR assays for the diagnosis of multidrug-resistant tuberculosis: a meta-analysis. Eur Respir J 32, 1165–1174 (2008).
Boehme, C. C. et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 363, 1005–1015 (2010).
Guo, Y. et al. Rapid, accurate determination of multidrug resistance in M. tuberculosis isolates and sputum using a biochip system. Int J Tuberc Lung Dis 13, 914–920 (2009).
World Health Organization. The use of molecular line probe assay for the detection of resistance to second-line anti-tuberculosis drugs. World Health Organization, Geneva, Switherland. WHO/HTM/TB/2013.01 (2013).
Hu, S. et al. Rapid detection of isoniazid resistance in Mycobacterium tuberculosis isolates by use of real-time-PCR-based melting curve analysis. J Clin Microbiol 52, 1644–1652 (2014).
Zhang, T. et al. Evaluation of the MeltPro TB/STR assay for rapid detection of streptomycin resistance in Mycobacterium tuberculosis. Tuberculosis (Edinb) 95, 162–169 (2015).
Ma, Y. et al. Evaluation of the mutations detection of rpoB gene in Mycobacterium tuberculosis clinical isolates by probe melting analysis based real-time PCR. Chinese Frontier Health Quarantine 34, 451–454 (2011).
Li, Q. et al. Multicenter Evaluation of the Molecular Line Probe Assay for Multidrug Resistant Mycobacterium Tuberculosis Detection in China. Biomed Environ Sci 28, 464–467 (2015).
Ou, X. et al. A feasibility study of the Xpert MTB/RIF test at the peripheral level laboratory in China. Int J Infect Dis 31, 41–46 (2015).
Chakravorty, S. et al. Rapid detection of fluoroquinolone-resistant and heteroresistant Mycobacterium tuberculosis by use of sloppy molecular beacons and dual melting-temperature codes in a real-time PCR assay. J Clin Microbiol 49, 932–940 (2011).
Zhang, X. et al. Subpopulation analysis of heteroresistance to fluoroquinolone in Mycobacterium tuberculosis isolates from Beijing, China. J Clin Microbiol 50, 1471–1474 (2012).
Zhang, X. et al. Co-occurrence of amikacin-resistant and -susceptible Mycobacterium tuberculosis isolates in clinical samples from Beijing, China. J Antimicrob Chemother 68, 1537–1542 (2013).
Chen, Q. et al. Molecular characteristics of MDR Mycobacterium tuberculosis strains isolated in Fujian, China. Tuberculosis (Edinb) 94, 159–161 (2014).
Mokrousov, I. et al. Molecular characterization of ofloxacin-resistant Mycobacterium tuberculosis strains from Russia. Antimicrob Agents Chemother 52, 2937–2939 (2008).
Huang, T. S. et al. Trends in fluoroquinolone resistance of Mycobacterium tuberculosis complex in a Taiwanese medical centre: 1995-2003. J Antimicrob Chemother 56, 1058–1062 (2005).
Zhang, Z., Wang, Y., Pang, Y. & Liu, C. Comparison of different drug susceptibility test methods to detect rifampin heteroresistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother 58, 5632–5635 (2014).
Zhang, D., Liu, B., Wang, Y. & Pang, Y. Rapid molecular screening for multidrug-resistant tuberculosis in a resource-limited region of China. Trop Med Int Health 19, 1259–1266 (2014).
Zhao, L. L. et al. Prevalence and molecular characteristics of drug-resistant Mycobacterium tuberculosis in Hunan, China. Antimicrob Agents Chemother 58, 3475–3480 (2014).
Rigouts, L. et al. Rifampin resistance missed in automated liquid culture system for Mycobacterium tuberculosis isolates with specific rpoB mutations. J Clin Microbiol 51, 2641–2645 (2013).
Koser, C. U. et al. Whole-genome sequencing for rapid susceptibility testing of M. tuberculosis. N Engl J Med 369, 290–292 (2013).
Ignatyeva, O. et al. Detection of resistance to second-line antituberculosis drugs by use of the genotype MTBDRsl assay: a multicenter evaluation and feasibility study. J Clin Microbiol 50, 1593–1597 (2012).
Zhang, Z., Lu, J., Wang, Y., Pang, Y. & Zhao, Y. Automated liquid culture system misses isoniazid heteroresistance in Mycobacterium tuberculosis isolates with mutations in the promoter region of the inhA gene. Eur J Clin Microbiol Infect Dis 34, 555–560, 10.1007/s10096-014-2262-0 (2015).
Zhang, Z. et al. Genotyping and molecular characteristics of multidrug-resistant Mycobacterium tuberculosis isolates from China. J Infect 70, 335–345 (2015).
Steingart, K. R. et al. Xpert MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 1, CD009593 (2013).
FIND. Non-inferiority Evaluation of Nipro NTM + MDRTB and Hain GenoType MTBDRplus V2 line probe assays. 4.1 (2015).
Acknowledgements
This work was supported by the Bill & Melinda Gates Foundation [2009-04-01]. We thank the local staffs from Guangzhou Chest Hospital and Shandong Chest Hospital for their hard work in this evaluation.
Author information
Authors and Affiliations
Contributions
Designed the studies: Y.P., H.D., Y.T., Y.D., Z.Z., J.L., J.Z., S.H. and Y.Z. Undertook the experimental work: Y.P., H.D., Y.T., Y.D., X.C., H.J., B.S. and X.L. Analysed the data: Y.P., H.D., H.X., Q.L., X.O., Z.Z., J.L. and Y.Z. Contributed to figure and manuscript preparation: Y.P., H.D., Y.T., Y.D. and Y.Z.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Pang, Y., Dong, H., Tan, Y. et al. Rapid diagnosis of MDR and XDR tuberculosis with the MeltPro TB assay in China. Sci Rep 6, 25330 (2016). https://doi.org/10.1038/srep25330
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep25330
This article is cited by
-
Performance of the MeltPro TB assay as initial test for diagnosis of pulmonary tuberculosis with drug-resistance detection
Molecular Medicine (2023)
-
Characteristics of rifampicin-resistant tuberculosis detection in China, 2015–2019
Infectious Diseases of Poverty (2021)
-
Whole genome sequencing of Mycobacterium tuberculosis isolates and clinical outcomes of patients treated for multidrug-resistant tuberculosis in Tanzania
BMC Genomics (2020)
-
Comparison of GeneXpert and line probe assay for detection of Mycobacterium tuberculosis and rifampicin-mono resistance at the National Tuberculosis Reference Laboratory, Kenya
BMC Infectious Diseases (2019)
-
An improved algorithm for rapid diagnosis of pleural tuberculosis from pleural effusion by combined testing with GeneXpert MTB/RIF and an anti-LAM antibody-based assay
BMC Infectious Diseases (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.