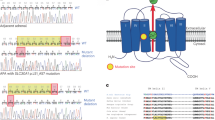
Abstract
Primary aldosteronism (PA) is the most common cause of secondary hypertension with a prevalence of 5–10% in unreferred hypertensive patients. Aldosterone producing adenomas (APAs) constitute a large proportion of PA cases and represent a surgically correctable form of the disease. The WNT signaling pathway is activated in APAs. In other tumors, a frequent cause of aberrant WNT signaling is mutation in the CTNNB1 gene coding for β-catenin. Our objective was to screen for CTNNB1 mutations in a well-characterized cohort of 198 APAs. Somatic CTNNB1 mutations were detected in 5.1% of the tumors, occurring mutually exclusive from mutations in KCNJ5, ATP1A1, ATP2B3 and CACNA1D. All of the observed mutations altered serine/threonine residues in the GSK3β binding domain in exon 3. The mutations were associated with stabilized β-catenin and increased AXIN2 expression, suggesting activation of WNT signaling. By CYP11B2 mRNA expression, CYP11B2 protein expression and direct measurement of aldosterone in tumor tissue, we confirmed the ability for aldosterone production. This report provides compelling evidence that aberrant WNT signaling caused by mutations in CTNNB1 occur in APAs. This also suggests that other mechanisms that constitutively activate the WNT pathway may be important in APA formation.
Similar content being viewed by others
Introduction
Primary aldosteronism (PA) is characterized by autonomous aldosterone production with increased aldosterone to renin ratio1. The increased level of aldosterone leads to abnormal retention of sodium and water and potassium excretion, causing hypervolemic hypertension and in more severe cases, hypokalemia2. Once thought to be a rare disease, PA is now being recognized as the most common cause of secondary hypertension with a prevalence of 5–10% in hypertensive individuals and up to 20% in therapy-resistant hypertension3,4,5,6. Most commonly, PA is either caused by unilateral aldosterone producing adenoma (APA) or bilateral adrenal hyperplasia (BAH)3,4. Unilateral APAs represent about 30% of PA cases3,4,5. Dominant unilateral aldosterone production caused by either APA or asymmetrical aldosterone release in the context of BAH can be cured by surgery, while patients with symmetrical BAH should be recommended mineralocorticoid receptor antagonists7. The importance of detecting PA patients among hypertensive individuals is not only due to the possibility for specific treatment and potential cure, but also because PA is an independent risk factor for increased cardiovascular morbidity, caused by excess aldosterone, independent of blood pressure8,9,10,11.
The WNT signaling pathway is important for normal development and maintenance of the adrenal cortex and more specifically, the zona glomerulosa (ZG) within the cortex12,13. Exon 3 of the CTNNB1 gene (encoding β-catenin), contains specific serine and threonine residues that, when phosphorylated, mark β-catenin for degradation14,15. Mutations affecting these amino acids, or deletions of exon 3, subsequently preclude phosphorylation of β-catenin, leading to aberrant activation of WNT signaling16,17,18,19,20.
Active WNT signaling has been observed in APAs21,22,23. The Secreted Frizzled-related protein II (SFRP2) is a negative regulator of the WNT pathway24 and its down-regulation has been proposed as one of the mechanisms for this increase in WNT signaling21. Mice with abnormal WNT signaling, due to alterations in exon 3 of the CTNNB1 gene, develop hyperaldosteronism and adrenocortical tumors23,25. Mutations in CTNNB1 also cause increased, abnormal WNT activation in human adrenocortical tumors26,27. Two independent exome sequencing experiments have demonstrated CTNNB1 mutations in two APAs28,29. The aim of this study was to determine the prevalence of CTNNB1 mutations in APAs.
Results
Mutation status of CTNNB1
The included APA samples exhibited mutations in KCNJ5 (n = 92, 46.5%), ATP1A1 (n = 6, 3.0%), ATP2B3 (n = 3, 1.5%), CACNA1D (n = 3, 1.5%), while 94 (47.5%) did not harbor mutations in known genes30,31. Somatic missense mutations in CTNNB1 were observed in 10 APAs (5.1% in the total cohort and in 10.6% of APAs without known mutations). No large deletions of exon 3 were observed in investigated non-mutated tumors (n = 7, Supplementary Fig. S1 online). The prevalence of CTNNB1 mutations was similar among different national cohorts (Supplementary Table S1 online). The detected mutations all occurred in conserved serine/threonine residues in exon 3 (Supplementary Fig. S2, Supplementary Table S2 online). The mutations were absent from constitutional DNA in all available specimens (n = 7), confirming their somatic nature. All mutations in CTNNB1 occurred mutually exclusive to KCNJ5, ATP1A1, ATP2B3 and CACNA1D (p = 1.47e-14, Fisher’s exact test).
Aldosterone production in CTNNB1 mutant tumors
We analyzed CYP11B2 mRNA expression by semi-quantitative RT-PCR in CTNNB1 mutated and KCNJ5 mutated tumors, as well as in NHPAs and CPAs. The investigated CTNNB1 mutants displayed higher CYP11B2 expression than NHPAs, CPAs and also significantly higher than tumors with KCNJ5 mutations (p = 0.011) (Fig. 1). CYP11B2 protein expression was analyzed by IHC using a mouse monoclonal antibody. Staining of normal adrenal tissue revealed heterogeneous expression selective for ZG (Fig. 2a). All investigated CTNNB1 mutated tumors (n = 8) exhibited expression of CYP11B2. Four had diffuse expression throughout the tumor (Fig. 2b), while four displayed limited heterogeneous staining (Fig. 2c). To further confirm the ability of CTNNB1 mutated tumors to produce aldosterone, aldosterone concentration were directly measured in tumor tissue lysates utilizing an automated immunoassay. The investigated CTNNB1 mutated APA lysates (n = 3) contained aldosterone at similar levels as KCNJ5 mutated tumor lysates (p = 0.48, Fig. 3).
CYP11B2 expression in APAs.
Relative mRNA expression of CYP11B2 in APAs with CTNNB1 mutation (n = 5) compared to APAs with KCNJ5 mutation (n = 16), NHPA (n = 4) and CPA (n = 4) using the 2(−ΔΔCT) method. GAPDH was used as the reference gene. Values indicate median with range. Y-axis shows logarithmic values. NHPA = Non-hormone producing adenoma. CPA = Cortisol producing adenoma. Statistical analysis using Mann -Whitney U-test.
CYP11B1/B2 protein expression.
Immunohistochemical analysis of CYP11B1/B2 expression. (a) CYP11B2 staining of normal adrenal gland. (b) CYP11B2 staining of a tumor with CTNNB1 mutation displaying diffuse expression. (c) CYP11B2 staining of a tumor with CTNNB1 mutation displaying weak heterogeneous expression. (d) CYP11B1 staining of normal adrenal gland. (e) CYP11B1 staining of a tumor with CTNNB1 mutation displaying weak heterogeneous expression. (f) CYP11B1 staining of a tumor with CTNNB1 mutation displaying diffuse expression.
Histologic appearance and CYP11B1/B2 protein expression in APAs with CTNNB1 and KCNJ5 mutation
Histologic examination of CTNNB1 mutated tumors did not reveal any specific cell morphology (Supplementary Table S3 online). CYP11B1 antibody specificity was confirmed in normal adrenal tissue (Fig. 2d). Staining of eight CTNNB1 mutated tumors revealed two distinct subgroups. In tumors with high CYP11B2 expression the CYP11B1 expression was also low (Fig. 2b,e). Similarly, in those with low CYP11B2 expression the CYP11B1 staining was strong (Fig. 2c,f). Comparing with KCNJ5 mutated APAs, no difference in CYP11B1 expression levels were observed, but a significantly higher CYP11B2 expression in CTNNB1 mutated tumors were observed (p = 0.02, Fig. 4a,b).
Comparison of CYP11B1/B2 expression score in APA.
Immunohistochemical expression score of CYP11B2 and CYP11B1 in tumors with CTNNB1 and KCNJ5 mutation.Y-axis display the expression score of CYP11B2, calculated using a modified H-score. (a) CYP11B2. (b) CYP11B1. Median with range. Statistical analysis using Mann-Whitney U-test.
β-catenin accumulation and activated WNT signaling in tumors with CTNNB1 mutation
All investigated APAs with CTNNB1 mutation displayed cytoplasmic and/or nuclear β-catenin staining (n = 8, Fig. 5) and displayed high levels of active β-catenin by western blot (n = 3, Fig. 6). No apparent nuclear staining was observed in tumors without CTNNB1 mutation, although 11/24 (45.8%) displayed cytoplasmic staining (H-score ≥50, Supplementary Table S4 and Supplementary Fig. S3 online). In the tumors without CTNNB1 mutation, β-catenin expression correlated with ZG-like cell content (r2 = 0.61, p < 0.0001, Supplementary Fig. S4 online). We did not observe any genotype-expression correlation as tumors with KCNJ5 mutation and ZG-like cell content displayed cytoplasmic expression of β-catenin (Supplementary Fig. S3 online). Western blot in one tumor with CTNNB1 mutation (p.Ser45Pro) and four without CTNNB1 mutation, validated antibody specificity for β-catenin (Supplementary Fig. S5 online). To analyze downstream targets of β-catenin, AXIN2 mRNA expression was measured. A significantly higher expression of AXIN2 in tumors with CTNNB1 mutation compared to those with KCNJ5 mutation was observed (p = 0.0029, Fig. 7). In five tumors without known mutations we observed high levels of AXIN2 suggesting active WNT signaling.
AXIN2 mRNA expression.
Relative mRNA expression of AXIN2 in APAs with CTNNB1 mutation (n = 5) compared with APAs with KCNJ5 mutation (n = 16), APAs with ATP1A1 and ATP2B3 mutation (n = 6) and seven APAs without known mutation, using the 2(−ΔΔCT) method. GAPDH was used as the reference gene. Values indicate median with interquartile range. Statistical analysis using Mann -Whitney U-test.
Clinical characteristics of patients with tumors harboring CTNNB1 mutation
The clinical data of the patients with tumors harboring CTNNB1 mutation are summarized in Supplementary Table S5 online. CTNNB1 mutated APAs were more often found in female patients (60%). There were no significant differences in preoperative aldosterone levels, tumor size, or age at surgery in patients with tumors harboring CTNNB1 mutation compared to those with tumors harboring KCNJ5 mutation (Supplementary Table S5 online). However a significant difference in size between the tumors with CTNNB1 mutation and tumors without known mutation was observed, median 24 mm (range 10–45) vs median 14 mm (range: 3–45, mean value 14.7 mm ± 7.9SD) (p = 0.01).
Discussion
WNT signaling is essential for normal development and maintenance of the adrenal cortex13. In the canonical WNT signaling pathway, absence of WNT ligands induces formation of a complex consisting of Dishevelled, AXIN, APC, CKI and GSK3β which binds and phosphorylates β-catenin, leading to its ubiquitination and subsequent degradation by proteasomes32. In contrast, WNT ligand binding to the frizzled and LRP co-receptors causes sequestering of the destruction complex at the cytoplasmic membrane, leaving it unable to phosphorylate. This leads to β-catenin accumulation, translocation to the nucleus and altered transcription of downstream targets, including: T-cell factor/lymphoid enhancer factor (TCF/LEF-1), CYP21, the Angiotensin I receptor and CYP11B221,33,34. β-catenin activation in the adrenal gland is normally restricted to cells of the ZG22. Stabilizing mutations in the CTNNB1 gene increase the activity of the finely tuned WNT signaling pathway, leading to tumor formation16. Previous reports have shown that mutations in β-catenin occur in a small proportion of APAs26,28,29,35. Yet contradictorily, two different studies sequencing APAs did not detect any CTNNB1 mutations21,22. Here we performed CTNNB1 sequencing in a large cohort of clinically and molecularly diagnosed APAs. We detected CTNNB1 mutations in 5.1% of cases, all affecting previously described mutation “hotspot” amino acids p.Thr41 and p.Ser45. All CTNNB1 mutations occurred mutually exclusive to KCNJ5, ATP1A1, ATP2B3 and CACNA1D mutated tumors, implying that aberrant WNT activation may be enough for APA formation as indicated in mouse models23. Tumors with CTNNB1 mutations were associated with relatively large adenomas and were found both in female and male patients, however with a small overrepresentation in females.
To analyze the effect on WNT signaling, we performed β-catenin IHC and quantitative PCR on AXIN2. We observed nuclear and/or cytoplasmic β-catenin staining in tumors with CTNNB1 mutation, indicating aberrant, increased protein accumulation. We also noted APAs without CTNNB1 mutation having cytoplasmic β-catenin expression and also those with relatively high mRNA expression of AXIN2. This suggests that WNT signaling is activated in a large subgroup of APAs and independent of genetic background22, raising the question if other genetic aberrations in the WNT pathway may be involved in development of APAs.
CYP11B2 and CYP11B1 are highly homologous enzymes expressed in ZF and ZG respectively. CYP11B1 converts 11-deoxycortisol to cortisol, whereas CYP11B2 converts deoxycorticosterone successively to corticosterone, 18-OH-corticosterone and finally aldosterone. CYP11B2 expression indicates aldosterone production and most APAs display expression, although in some cases heterogeneously36,37. Utilizing specific mRNA probes and primers for CYP11B238, we found expression of CYP11B2 mRNA in tumors with CTNNB1 mutations indicating ability to produce aldosterone. IHC utilizing an antibody against CYP11B2 showed high specificity for ZG. We verified expression of CYP11B2 in all CTNNB1 tumors. Four CTNNB1 mutated tumors demonstrated diffuse expression, while four tumors exhibited heterogeneous and relatively weak staining, similar to the pattern seen in many of the tumors with KCNJ5 mutation36,37. To further confirm aldosterone production, we measured aldosterone concentration in tumor lysates using a chemiluminescent immunoassay. The investigated mutants contained aldosterone and at similar levels to other APAs with KCNJ5 mutations indicating an active aldosterone production. Measuring aldosterone in tumor tissue may provide a diagnostic tool to discriminate functional aldosterone producing nodules from non-functional and with introduction of faster assays, could even be employed in the operating theatres.
APAs may contain a varying degree of ZG-like cells and ZF-like cells39. Some reports indicate that this phenotype depends on underlying genetic alteration29,37,40, whereas others do not41. Also, a difference in the protein expression pattern of CYP11B2/B1 has been observed between the different mutants36,37. By histologic examination, we observed a variable histologic appearance in CTNNB1 mutated tumors. Interestingly, we observed two subgroups of tumors; one with diffuse CYP11B2 expression with concomitantly low CYP11B1 expression and one with low CYP11B2 and high CYP11B1 expressions. Perhaps suggesting that these mutations mainly promote proliferation.
CTNNB1 mutations occur in cortisol producing adenomas (CPA). Recently, mutations in the catalytic subunit of Protein kinase A (PKA) were identified and shown to occur mutually exclusive to CTNNB1 mutations42,43,44,45. Activating mutations in PKA lead to a constitutively activated cAMP signaling, causing increased cortisol production and likely tumor formation. Expression analysis revealed increased expression of genes involved in biosynthesis and metabolism of steroids in tumors with PRKACA mutation42. This suggests two pathways for CPA formation: either alterations in cAMP signaling with increased steroidogenesis, or other mutations mainly stimulating proliferation. In APAs, most mutations observed affect the membrane potential and increase the likelihood of depolarization. These tumors harbor few mutations per megabase and seldom have CNV alterations28. In contrast, many APAs without known mutation have CNV alterations28, suggesting different pathways for APA development, similar to CPAs. We propose that CTNNB1 mutations belong to a group of alterations mainly affecting proliferation rate. The low prevalence of mutations in APAs compared to NHPAs or CPAs could reflect the low number of ZG cells expressing CYP11B2 in normal adrenal gland.
It is unknown if carcinomas of the adrenal cortex evolve in an adenoma-to-carcinoma sequence46. Evidence in favor of this hypothesis comes from experiments using β-catenin mutated mice that develop benign tumors that can progress to malignancy, suggesting requirement of additional genetic alterations23,25. This is in line with the multistep progression model seen in patients with familial adenomatous polyposis47. Moreover, signs of carcinomas inside benign adrenal masses have been described48,49,50. However, most APAs rarely increase in size and aldosterone producing carcinomas are exceedingly rare7. If adenoma to carcinoma sequences does occur, one could speculate that patients with tumors harboring CTNNB1 mutation have a small but increased risk of malignant transformation. Another interesting observation, coming from the β-catenin stabilized mouse model is that up to 10 months of age the mice produce aldosterone and show no signs of corticosterone production. Despite this, 17-month-old mice exhibit a trend towards lower aldosterone synthesis and increased corticosterone production, suggesting that the level of WNT activity determine the functional characteristics of the lesion23. This could also explain why mutations in CTNNB1 are less frequent in APAs.
In conclusion, we observed CTNNB1 mutations in a subset of APAs with aberrant β-catenin accumulation. Tumors harboring these mutations have a variable histological and CYP11B2/B1 expression pattern, produce aldosterone and show similar clinical characteristics as patients with tumors harboring KCNJ5 mutation. We speculate that CTNNB1 mutations lead to a proliferative advantage in the adrenal cell it occurs in. Because of the higher frequency of WNT activation in APAs than CTNNB1 mutations, future investigations of alterations in other members of the WNT pathway are warranted.
Materials and Methods
Tissues
Adrenocortical tumor tissues were collected from six different centers. According to routine protocols at the centers, a diagnosis of APA was established by these criteria: an elevated aldosterone/renin ratio together with positive confirmatory tests and lateralization studies by CT, MRI and/or adrenal vein sampling and a postoperative cure or considerable improvement. Patients with cortisol producing adenomas (CPA) had elevated serum cortisol, failure to suppress cortisol with dexamethasone, normal aldosterone levels and all were postoperatively cured. Non-hormone producing adenomas (NHPA) were detected en passant by abdominal CT scan in biochemically normal and asymptomatic patients and were removed due to large size or malignant features on CT.
DNA and RNA extraction
DNA and RNA from frozen tissues were extracted using Allprep DNA/RNA kit, (Qiagen, Hilden, Germany) or FFPE tissue using AllPrep DNA/RNA FFPE Kit (Qiagen, Hilden, Germany) and from blood using DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany). Synthesis of cDNA was performed using the First-Strand cDNA Synthesis kit according to the manufacturer’s instructions (Fermentas, Thermo Fisher Scientific, Waltham, USA).
PCR amplification and DNA sequencing
Exon 3 of the CTNNB1 gene was amplified by PCR amplification, using primers: FW: 5′-TGTCTTTCAGATTTGACTTTATTT, RW: 5′-TCAAAACTGCATTCTGACTTTCA followed by a nested reaction with FW2: 5′-CAATGGGTCATATCACAGATTCTT, then Sanger sequenced. Traces were analyzed and compared to the CTNNB1 reference sequence NP_001895.1. Observed mutations were validated in an independent PCR reaction. cDNA was sequenced using mRNA specific primers, cDNA FW: 5′-GCTACTCAAGCTGATTTGATG and RW: 5′-CAGGACTTGGGAGGTATCC. Large deletions affecting exon 3 of the CTNNB1 gene were investigated by RT-PCR amplification using mRNA specific primers, FW primer: 5′-GAAGGTCTGAGGAGCAGCTTC and RW: 5′-GACATTAGATGAGGGCATGC. The obtained amplicons were run on a 2% agarose gel to visualize size of the products. All tumors had previously been sequenced for KCNJ5, ATP1A1, ATP2B3 and CACNA1D mutations31.
Histology examination and immunohistochemistry
Tumors were evaluated to determine their zona glomerulosa (ZG) and zona fasciculata (ZF) cell content. Cells were classified as ZG-like if they had a high nuclear to cytoplasmic ratio and formed circular cell clusters. Cells were classified as ZF-like if they were lipid-rich with a high cytoplasm to nuclear ratio and a more uniform sponge-like phenotype39. IHC for β-catenin was conducted on eight tumors with CTNNB1 mutation and 24 tumors without CTNNB1 mutation, using an anti-β-catenin goat polyclonal antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA; catalog no. sc-1496, dilution 1:5) as previously described18. Staining of CYP11B1 and CYP11B2 were conducted using rat and mouse monoclonal antibodies respectively51. Briefly, 5 μm sections were deparaffinized in xylene and rehydrated through graded ethanol. Endogenous peroxidases were inhibited by incubation in hydrogen peroxide solution. Antigen retrieval using 10 mM sodium citrate (pH6.0) was used. Sections were then incubated with 10% normal goat serum/horse serum after which the primary antibody was added (CYP11B1, 1:100 and CYP11B2, 1:250) overnight. For detection, biotinylated HRP conjugated goat anti-rat or horse anti-mouse secondary antibodies were used, 1:200 (Vector Laboratories, Burlingame, USA). The sections were developed with DAB (Vector Laboratories, Burlingame, USA) and counterstained with hematoxilin. Two researchers, unaware of the mutational status, conducted comparison of expression. A scoring system using a modified H-score was applied52. Five 20× fields and the entire sample were scored on intensity i (0 = negative, 1 = weak but detectable, 2 = distinct, 3 = strong) and percentage of stained cells Pi. A mean value of these six measurements was used in the formula ∑ = Pi(i + 1). Images were acquired using a Zeiss Observed Z1 microscope (Zeiss, Oberkochen, Germany) and imported using the AxioVision SE64 4.9.1 software (Zeiss, Oberkochen, Germany).
Western blot
To verify specificity of the β-catenin antibody used in the IHC experiment, western blot analysis was performed on one CTNNB1 mutated APA (p.Ser45Pro) and four APAs without CTNNB1 mutation (Santa Cruz-1496; dilution 1:1000), with anti-actin (Santa Cruz-1616) as loading control. Western blot was also performed on three tumors with CTNNB1 mutation, two with KCNJ5 mutation and one APA without known mutation, using an anti-active β-catenin antibody (Millipore 05–655; dilution 1:1000) and anti-actin (Santa Cruz-1616) as loading control.
Semiquantitative RT-PCR
The expression of CYP11B2 was measured using an mRNA specific custom assay (ThermoFisher Scientific, Waltham, USA)38. AXIN2 mRNA expression was quantified using SYBR green with FW primer: 5′-GGTCCACGGAAACTGTTGACA and RW: 5′-GGCTGGTGCAAAGACATAGCC. An mRNA specific GAPDH assay was used as internal control (4352934, ThermoFisher Scientific, Waltham, USA). The expression was measured in duplicates and a relative difference was obtained using the 2(−ΔΔCT) method53.
Chemiluminescent immunoassay
Sections (20 μm) of frozen tumor tissue from three tumors with CTNNB1 mutations, six with KCNJ5 mutation and one NHPA, were weighed with a highly sensitive scale (±0.01 mg) and homogenized in filtered water. The lysate was again weighed and a concentration of mg of tumor tissue per mg of water was calculated. The samples were then centrifuged for 20 minutes at 20000 RPM. The obtained supernatant was diluted 1:10 and subjected to an aldosterone measurement using a chemiluminescent immunoassay (DiaSorin, Italy) used in routine clinical practice at the Department of Clinical Chemistry at Uppsala University hospital. A concentration in pmol/L was obtained and adjusted to the concentration. This gave a concentration of aldosterone/mg tissue (pmol/mg).
Statistics
The overall group effect was analyzed using a Kruskal-Wallis test. If significant group effects were present the overall analysis was followed by post-hoc comparisons between groups using a Mann-Whitney U-test with Bonferroni correction. Categorical data were analyzed by Chi-squared tests. Data are expressed as either mean ± SD or median and range for non-parametric data. For statistical analysis SPSS 22 (IBM, NY, USA) was used.
Additional Information
How to cite this article: Åkerström, T. et al. Activating mutations in CTNNB1 in aldosterone producing adenomas. Sci. Rep. 6, 19546; doi: 10.1038/srep19546 (2016).
References
Hamlet, S. M., Tunny, T. J., Woodland, E. & RD., G. Is aldosterone/renin ratio useful to screen a hypertensive population for primary aldosteronism? Clin Exp Pharmacol Physiol. 12, 249–252 (1985).
Conn, J. W., Knopf, R. F. & Nesbit, R. Clinical characteristics of primary aldosteronism from an analysis of 145 cases. Am J Surg 107, 159–172 (1964).
Mulatero P. et al. Increased Diagnosis of Primary Aldosteronism, Including Surgically Correctable Forms, in Centers from Five Continents. Journal of Clinical Endocrinology & Metabolism 89, 1045–1050 (2004).
Fogari, R. et al. Prevalence of primary aldosteronism among unselected hypertensive patients: A prospective study based on the use of an aldosterone/renin ratio above 25 as a screening test. Hypertension Research 30, 111–117 (2007).
Rossi, G. P. et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol 48, 2293–2300 (2006).
Hannemann, A. et al. Screening for primary aldosteronism in hypertensive subjects: results from two German epidemiological studies. Eur J Endocrinol 167, 7–15 (2012).
Funder, J. W. et al. Case detection, diagnosis and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. The Journal of clinical endocrinology and metabolism 93, 3266–3281 (2008).
Stowasser, M. et al. Evidence for abnormal left ventricular structure and function in normotensive individuals with familial hyperaldosteronism type I. The Journal of clinical endocrinology and metabolism 90, 5070–5076 (2005).
Rossi, G. P. et al. Changes in Left Ventricular Anatomy and Function in Hypertension and Primary Aldosteronism. Hypertension 27, 1039–1045 (1996).
Milliez, P. et al. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol 45, 1243–1248 (2005).
Savard, S., Amar, L., Plouin, P. F. & Steichen, O. Cardiovascular Complications Associated With Primary Aldosteronism: A Controlled Cross-Sectional Study. Hypertension 62, 331–336 (2013).
Heikkila, M. Wnt-4 Deficiency Alters Mouse Adrenal Cortex Function, Reducing Aldosterone Production. Endocrinology 143, 4358–4365 (2002).
Kim, A. C. et al. Targeted disruption of beta-catenin in Sf1-expressing cells impairs development and maintenance of the adrenal cortex. Development 135, 2593–2602 (2008).
Klinensmith, J. & Nusse, A. R. Signaling by wingless in Drosophilia. Developmental biology 166, 396–414 (1994).
Liu C. et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108, 837–847 (2002).
Morin, P. J. Activation of beta -Catenin-Tcf Signaling in Colon Cancer by Mutations in beta -Catenin or APC. Science 275, 1787–1790 (1997).
Rubinfeld, B. Stabilization of beta -Catenin by Genetic Defects in Melanoma Cell Lines. Science 275, 1790–1792 (1997).
Bjorklund, P., Lindberg, D., Akerstrom, G. & Westin, G. Stabilizing mutation of CTNNB1/beta-catenin and protein accumulation analyzed in a large series of parathyroid tumors of Swedish patients. Mol Cancer 7, 53 (2008).
Polakis, P. Wnt signaling and cancer. Genes Dev., 1837–1851 (2000).
Iwao K. et al. Activation of the beta-catenin gene by interstitial deletions involving exon 3 in primary colorectal carcinomas without adenomatous polyposis coli mutations. Cancer Research 58, 1021–1026 (1998).
Annabel Berthon et al. WNT/beta-catenin Signalling is Activated in Aldosterone Producing Adenomas and Controls Aldosterone Production. Hum. Mol. Genet 23, 889–905 (2014).
Boulkroun, S. et al. Aldosterone-producing adenoma formation in the adrenal cortex involves expression of stem/progenitor cell markers. Endocrinology 152, 4753–4763 (2011).
Berthon, A. et al. Constitutive beta-catenin activation induces adrenal hyperplasia and promotes adrenal cancer development. Hum Mol Genet 19, 1561–1576 (2010).
Wawrzak, D. et al. Wnt3a binds to several sFRPs in the nanomolar range. Biochem Biophys Res Commun 357, 1119–1123 (2007).
Heaton J. H. et al. Progression to Adrenocortical Tumorigenesis in Mice and Humans through Insulin-Like Growth Factor 2 and Beta-Catenin. Am J Pathol. 181, 1017–1033 (2012).
Durand, J., Lampron, A., Mazzuco, T. L., Chapman, A. & Bourdeau, I. Characterization of differential gene expression in adrenocortical tumors harboring beta-catenin (CTNNB1) mutations. The Journal of clinical endocrinology and metabolism 96, E1206–1211 (2011).
Tissier, F. et al. Mutations of beta-catenin in adrenocortical tumors: activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res 65, 7622–7627 (2005).
Scholl, U. I. et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet 45, 1050–1054 (2013).
Azizan, E. A. et al. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet 45, 1055–1060 (2013).
Akerstrom, T. et al. Comprehensive Re-Sequencing of Adrenal Aldosterone Producing Lesions Reveal Three Somatic Mutations near the KCNJ5 Potassium Channel Selectivity Filter. PloS one 7, e41926 (2012).
Tobias Åkerström et al. Novel somatic mutations and distinct molecular signature in aldosterone-producing adenomas. Endocr Relat Cancer. 5, 735–744 (2015).
Chen, X., Yang, J., Evans, P. M. & Liu, C. Wnt signaling: the good and the bad. Acta Biochimica et Biophysica Sinica 40, 577–594 (2008).
Brunner, E., Peter, O., Schweizer, L. & Basler, K. Pangolin encodes a LEF-1 homologue that acts downstream of Armadillo to transduce the Wingless signal in Drosophilia. Nature 385, 829–833 (1997).
Marc van de Wetering et al. Armadillo Coactivates Transcription Driven by the Product of the Drosophila Segment Polarity Gene dTCF. Cell 88, 789–799 (1997).
Tadjine, M., Lampron, A., Ouadi, L. & Bourdeau, I. Frequent mutations of beta-catenin gene in sporadic secreting adrenocortical adenomas. Clin Endocrinol (Oxf) 68, 264–270 (2008).
Silvia Monticone et al. Immunohistochemical, genetic and clinical characterization of sporadic aldosterone-producing adenomas. Molecular and Cellular Endocrinology 411, 146–154 (2015).
Dekkers, T. et al. Adrenal Nodularity and Somatic Mutations in Primary Aldosteronism: One Node Is the Culprit? The Journal of clinical endocrinology and metabolism 99, E1341–1351 (2014).
F. Fallo et al. Quantitative assessment of CYP11B1 and CYP11B2 expression in aldosterone-producing adenomas. Eur J Endocrinol. 147, 795–802 (2012).
A. M. Neville, C. & Symington, T. Pathology of primary aldosteronism. Cancer 19, 1854–1868 (1966).
Azizan, E. A. et al. Microarray, qPCR and KCNJ5 sequencing of aldosterone-producing adenomas reveal differences in genotype and phenotype between zona glomerulosa- and zona fasciculata-like tumors. The Journal of clinical endocrinology and metabolism 97, E819–829 (2012).
Fernandes-Rosa, F. L. et al. Genetic Spectrum and Clinical Correlates of Somatic Mutations in Aldosterone-Producing Adenoma. Hypertension 64, 354–361 (2014).
Cao, Y. et al. Activating hotspot L205R mutation in PRKACA and adrenal Cushing’s syndrome. Science 344, 913–917 (2014).
Beuschlein, F. et al. Constitutive activation of PKA catalytic subunit in adrenal Cushing’s syndrome. N Engl J Med 370, 1019–1028 (2014).
Goh, G. et al. Recurrent activating mutation in PRKACA in cortisol-producing adrenal tumors. Nat Genet 46, 613–617 (2014).
Sato, Y. et al. Recurrent somatic mutations underlie corticotropin-independent Cushing’s syndrome. Science 344, 917–920 (2014).
Fearon E. R. & B., V. A genetic model for colorectal tumorigenesis. Cell 61, 759–767 (1990).
Phelps, R. A. et al. A two-step model for colon adenoma initiation and progression caused by APC loss. Cell 137, 623–634 (2009).
Marie-Hélène Bernard et al. A Case Report in Favor of a Multistep Adrenocortical Tumorigenesis JCEM 88, 998–1001 (2003).
Rossella Libé et al. Adrenocortical Tumor with Two Distinct Elements Revealed by Combined 18F-Fluorodeoxyglucose Positron Emission Tomography and 131I Nor-Cholesterol Scintigraphy JCEM 94, 3631–3632 (2009).
Gaujoux, S. et al. Wnt/beta-catenin and 3′,5′-cyclic adenosine 5′-monophosphate/protein kinase A signaling pathways alterations and somatic beta-catenin gene mutations in the progression of adrenocortical tumors. The Journal of clinical endocrinology and metabolism 93, 4135–4140 (2008).
Gomez-Sanchez, C. E. et al. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol Cell Endocrinol 383, 111–117 (2014).
Vedovato, N. & Gadsby, D. C. Route, mechanism and implications of proton import during Na+/K+ exchange by native Na+/K+-ATPase pumps. The Journal of general physiology 143, 449–464 (2014).
Christian, A., Heid, J. S., Kenneth J., Livak & Mickey, P. Williams Real time quantitative PCR. Genome Res. 6, 986–994 (1996).
Acknowledgements
We would like to thank Birgitta Bondeson for all help and guidance. A special thank you to Drs Joakim Crona, Dominic-Luc Webb and Elena Azizan for critical revision of this manuscript. Finally, we would like to acknowledge all the patients included in this study. This work was supported by grants from the Swedish Cancer Society, Swedish Research Council, Selander Foundation and LIONS cancer research fund. PB is a Swedish Cancer Society Investigator.
Author information
Authors and Affiliations
Contributions
T.Å. and R.M. performed experiments. T.Å. carried out statistical analysis and drafted the manuscript. T.Å., R.M., E.A., H.S.W., K.C., J.I., A.M., B.R., K.A.I., H.D., M.K.W., H.L., S.S., C.G.S., P.H. and P.B. participated in the acquisition, analysis, or interpretation of data. All authors contributed with critical revision of the manuscript and all authors performed administrative, technical or material support. P.B. and P.H. obtained funding. T.Å. and P.B. designed the study. P.B. conceived and supervised the study.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Åkerström, T., Maharjan, R., Sven Willenberg, H. et al. Activating mutations in CTNNB1 in aldosterone producing adenomas. Sci Rep 6, 19546 (2016). https://doi.org/10.1038/srep19546
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep19546
This article is cited by
-
Molecular pathology of endocrine gland tumors: genetic alterations and clinicopathologic relevance
Virchows Archiv (2023)
-
Overview of the 2022 WHO Classification of Adrenal Cortical Tumors
Endocrine Pathology (2022)
-
Adrenal cortex renewal in health and disease
Nature Reviews Endocrinology (2021)
-
What Did We Learn from the Molecular Biology of Adrenal Cortical Neoplasia? From Histopathology to Translational Genomics
Endocrine Pathology (2021)
-
Somatic mutations of GNA11 and GNAQ in CTNNB1-mutant aldosterone-producing adenomas presenting in puberty, pregnancy or menopause
Nature Genetics (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.