Abstract
Current evidence suggests that beta-blocker lower the risk of development of atrial fibrillation (AF) and in-hospital stroke after cardiac surgery. This study was to assess whether beta-blockers could decrease incidence of new-onset AF in patients with end stage renal disease (ESRD). We identified patients from a nation-wide database called Registry for Catastrophic Illness, which encompassed almost 100% of the patients receiving dialysis therapy in Taiwan from 1995 to 2008. Propensity score matching and Cox’s proportional hazards regression model were used to estimate hazard ratios (HRs) for new-onset AF. Among 100066 patients, 41.7% received beta-blockers. After a median follow-up of 1500 days, the incidence of new-onset AF significantly decreased in patients treated with beta-blockers (HR = 0.483, 95% confidence interval = 0.437-0.534). The prevention of new-onset AF was significantly better in patients taking longer duration of beta-blockers therapy (P for time trend <0.001). The AF prevention effect remains robust in subgroup analyses. In conclusion, beta-blockers seem effective in the primary prevention of AF in ESRD patients. Hence, beta-blockers may be the target about upstream treatment of AF.
Similar content being viewed by others
Introduction
Atrial fibrillation (AF) is a result of continuous remodeling of the atrial, a dynamic interaction between a trigger and the substrate1,2. AF is increasingly associated with hypertension (HTN), congestive heart failure (CHF), diabetes mellitus (DM) and chronic kidney disease, all of which are recognized risk factors for the arrhythmia3,4,5. It is also prevalent after surgery, particular cardiothoracic surgery6. Postoperative AF (POAF) is likely related to pre-existing degenerative change in the atrial myocardium and perioperative conditions that result in abnormal electrophysiologic properties7,8. Potential adverse outcomes following postoperative AF include stroke, prolongation of hospital stay and death910.
Beta-blockers administration is the most widely used prophylactic strategy of POAF based on numerous studies showing benefit, ease of use and cost consideration11,12. The present guideline also recommends preoperative or early postoperative administration of beta-blockers in patients without contraindication in order to reduce the incidence of AF and clinical sequels after coronary bypass surgery13. This prophylactic therapy for POAF targets the sympathetic nervous system, atrial refractory period and conduction even though the mechanism of POAF is likely multifactorial8,14.
Patients with chronic kidney disease and end-stage renal disease (ESRD) are more likely to develop cardiovascular disease, including myocardial infarction15, sudden cardiac death16 and AF17,18. With regard to AF, poor controlled HTN and expansion of body fluid related to renal dysfunction lead to atrial stretch and fibrosis19. Pathological activation of renin-angiotensin-aldosterone system and systemic inflammation also create the required substrate for development of AF20,21. Although it is not clear whether patients with perioperative status and ESRD share common etiologies for AF, but both conditions have many similar factors prone to the development of AF.
In the AFFIRM study, beta-blockers were the most effectively and commonly used drug class for rate control22. Current guidelines recommend beta-blockers as one of rate control drugs, especially useful in the presence of high adrenergic tone or myocardium ischemia23,24. However, there is a paucity of studies concerning the role of beta-blockers on prophylactic effect for AF in ESRD patients. The present study was undertaken to assess the impact of treatment with beta-blockers on the development of AF in a large cohort of ESRD patients. We hypothesized that patients receiving beta-blockers would be associated with lower AF risks.
Results
Patient characteristics
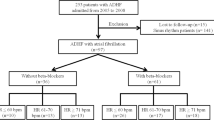
There were 100066 patients who met the study inclusion criteria; 58382 (58.3%) did not use beta-blockers while 41684 (41.7%) used beta-blockers. Patients not receiving beta-blocker treatment were served as control group. The median follow-up time was 1500 days. The algorithm was listed in Fig. 1.
Clinical and demographic characteristics were listed in Table 1. Patients without beta-blocker treatment were significantly elder than those with beta-blocker therapy and there were also significantly less female patients in non-beta-blocker group. The prevalence of receiving hemodialysis therapy was significantly higher in beta-blocker group (98.5%) than in control group (85.6%). As expected, the prevalence of risk factors including HTN (91.5% vs. 87.8%), DM (50.4% vs. 40.9%) and dyslipidemia (4.9% vs. 27.8%) were higher in beta-blocker group as well. The prevalence of comorbidities including ischaemic stroke/TIA (7.7% vs. 4.1%), hemorrhagic stroke (6.0% vs. 3.9%), CAD (50.2% vs. 29.2%), PAD (28.2% vs. 21.9%) and CHF hospitalization (27.2% vs. 21.3%) was also higher in beta-blocker group than in control group. Among the medication use, as compared with control group, ACEI/ARB (44.5% vs. 17.8%), calcium channel blockers (CCBs) (53.8% vs. 31.2%), diuretics (39.2% vs. 33.8%), statin (31.3% vs. 25.9%), OADs (29% vs. 23.3%) and insulin (19.2% vs. 7.3%) were more common in beta-blocker group. To minimize differences in the baseline characteristics between beta-blocker and non-beta-blocker group, patients were matched by using propensity method. As shown in Table 1, a total of 83340 patients were selected by propensity matching, the basic characteristics were matched well except in the use of ACEI/ARB and insulin.
Main outcome: AF
The median durations of follow-up were 1295 and 1741 days in control and in beta-blocker group. As demonstrated in Table 2, the absolute incidence of new-onset AF during the entire follow-up period was less in beta-blockers group (1.5%) as compared with that in control group (4.9%) (Table 2). After transforming the incidence into patient-years, the incidence was still higher in control group (9.3 per 1000 patient-years) than in beta-blocker group (2.7 per 1000 patient-years).
The results of Cox’s regression analyses were demonstrated in Table 3. After adjusting for potential confounders, in comparison with the control group, use of beta-blockers (Model 1; adjusted HR, 0.483; 95% CI: 0.437-0.534) was associated with lower risk for developing AF. We observed similar results after propensity matching (Model 2; adjusted HR, 0.426; 95% CI: 0.347-0.512) and after further adjustment with propensity score (Model 3; adjusted HR, 0.488; 95% CI: 0.441-0.541)(Table 3). We stratified the treatment duration of beta-blockers into three categories including ≦60 days, 60-180 days and >180 days. For patients treated with beta-blockers, the adjusted HR were 0.559 (95% CI: 0.467-0.670), 0.472 (95% CI: 0.407-0.546) and 0.457 (95% CI: 0.398 to 0.524), respectively (Table 3). There was a dose-response relationship for duration of treatment and the prevention of new-onset AF (P < 0.05).The finding remained unchanged after propensity matching and adjustment. The Kaplan-Meier survival curves were illustrated in Fig. 2. We plotted survival curves based on crude population and matching population. The log-rank test was significant in beta-blocker vs. control group (P < 0.001).
The results of subgroup analyses were demonstrated in Fig. 3. As shown in Fig. 3, the result that beta-blockers usage was associated with lower risk of new- onset AF remained unchanged in all subgroups (different age class, gender, HTN, DM, cardiovascular disease and CHF).
Discussions
Currently, accepted primary prevention of AF was discouragingly limited. According to ESC 2012 guidelines, recent double-blind, placebo-controlled trials with ARBs and the majority of trials with polyunsaturated fatty acids failed to show convincing results25. Though some evidence existed for specific patient groups, such as ARB for heart failure patients26, patients with severe renal insufficiency were usually excluded from those studies27,28,29. In the meantime, patients with ESRD generally suffered from greater burden of AF, but primary prevention was less mentioned in the literature. The main finding from this nationwide database retrospective study of patients with ESRD is the protective effect of beta-blocker against development of AF, with an HR around 0.4 to 0.6 if the patient had received beta-blocker. A considerable percentage of the study population had other comorbidities, but consistent association between beta-blocker use and reduced incidence of AF was demonstrated from subgroup analysis. This might imply direct protective effect of beta-blocker in patient with ESRD, instead of simply treating the underlying comorbidities. The beneficial effect of beta-blocker was consistent irrespective of patients’ age, underlying comorbidities and even in patients treated with beta-blocker less than 2 months. Therefore, beta-blocker might potentially be an effective therapy for primary prevention in patients with ESRD.
Impact of AF on ESRD
AF, while being the most common arrhythmia among general population, is also the most common arrhythmia among patients with ESRD16, accounting for 13% of patients on hemodialysis and 7% in patients undergoing peritoneal dialysis according to report in the large US Renal Data System (USRDS)30. Compared to general population, patients with ESRD suffer from even greater burden of AF and with population aging, the numbers of patients with concomitant ESRD and AF will increase in the future31. The reason for more AF in patients with ESRD had been a topic receiving great attention in the past decades.
Both increased comorbid illnesses and non-traditional factors, such as inflammation and oxidative stress, contribute to the formation of substrate of AF in patients with ESRD32,33,34. Altered cardiac chambers dimensions and function were also in favor of AF development35. Compared to patients without AF, patients who developed AF had increased 4-year mortality and risk of stroke36. Contemporary guidelines has well-established recommendations regarding timing of anticoagulation, based on CHA2DS2VASc score, but currently there is insufficient evidence to support application of these recommendations to patients with ESRD. From prior study, warfarin was associated with inconsistent results in patients with ESRD and was even a risk factor for stroke37. Furthermore, use of warfarin in patients with ESRD could double the risk of bleeding shown by two retrospective studies38,39. Given the clinical consequence, primary prevention of AF in patients with ESRD is of great importance.
Primary prevention in AF
In the field of AF primary prevention, namely upstream therapy, effective therapy was discouragingly limited. ACEI/ARB did show its preventive effect in patients with HF with reduced LV ejection fraction from some retrospective studies40,41,42; however, this effect could not be reproduced in patients without structural heart disease in the Angiotensin II-antagonist in paroxysmal atrial fibrillation (ANTIPAF) trial43. In Japanese Rhythm Management Trial II for Atrial Fibrillation (J-RHYTHM II study), candesartan did not show its advantage over amlodipine in reducing AF frequency44. Statin was well known for the pleiotropic effect in cardiovascular disease, but meta-analysis showed that it could only reduce the risk of AF after coronary surgery45.
Several other pathophysiological processes, including sympathetic activation and oxidative stress, are associated with atrial remodeling that may predispose patients to AF and could also be the targets of upstream therapy46. A representative example is beta-blockade in POAF. Given the condition with increased sympathetic tone and loss of vagal tone before POAF occurrence47 and the example of successful induction of AF by injecting isoproterenol and adrenaline into the sinus node artery in animal study48, beta-blockade reasonably became a therapeutic choice in preventing POAF. In a randomized control study, beta-blockade with short-term intravenous landiolol and long-term oral bisoprolol reduced up to 70% of POAF compared to control group49. To go a step further, biomarker tests demonstrated anti-ischemic, anti-inflammatory and anti-oxidant effects brought by beta-blockade.
Patients with ERSD are also a special group in a milieu of high baseline sympathetic tone and oxidative stress33,50,51 and a retrospective study would serve well as the initial evaluation of potential benefit of beta-blockade. In addition to beta-blockade, prevent atrial substrate formation through RAAS-blockade is another therapeutic target since RAAS activation is also a frequent finding in patients with renal impairment52. Recently, our study group had found ACEI/ARB to be effective in reducing new-onset AF in patients with ESRD53, potentially indicating more contribution from RAS activation. This again emphasized on the unique pathophysiological characters and different therapeutic considerations of AF in patients with ESRD.
Limitations
There are several limitations needed to be addressed. First, the study is limited by its retrospective, nonrandomized nature and the imbalance in risk factors among different antihyperglycemic agents’ users in the whole cohort. Also, treatment allocation was not randomized and treatment selection bias had to be taken into account. Although we adjusted the confounding factors, the result might be still confounded by other underlying disease we did not consider. Second, our primary end-point was the time to the occurrence of AF diagnosed via ICD-CM coding. The accuracy of the diagnoses, which are based on the administrative data reported by physicians, may be a concern. Patients prescribed with beta-blocker might benefit more from beta-blocker originally, for example, more HF with reduced EF, which cannot be discriminated from patients with HF with preserved EF from the database. There is a risk that much AF is underreported because of diagnostic modality being used. Third, although we adjusted confounding factors, the result might be still confounded by other underlying disease we did not consider. Finally, we did not distinguish between chronic and paroxysmal AF and the results might differ in these two conditions.
Conclusions
In patients with ESRD receiving replacement therapy, beta-blocker usage is associated with appeared to be effective in the primary prevention of AF.
Materials and Methods
Source of data
This large-scale, longitudinal cohort study used integrated medical and pharmacy claims data from National Health Insurance Research Database (NHIRD) in Taiwan. The National Health Insurance program has provided compulsory universal health insurance in Taiwan since 1995. More than 98% of the total Taiwanese population of 23 million is covered by the program. The NHIRD contains nearly complete claims history of diagnosis and procedures, provided as the International Classification of Diseases Ninth Revision Clinical Modification (ICD-9-CM) codes and drug dispensing for every beneficiary. The NHIRD established a registry system for “Catastrophic Illnesses”, including cancer, chronic mental illness, congenital illness and ESRD. In Taiwan, ESRD patients undergoing hemodialysis and peritoneal dialysis are categorized as “severe illness” and the national insurance covers almost all the medical fees. All the medications, procedures, every out-patient clinic visits and hospital admission covered by the insurance were recorded in the database. The Bureau of National Health Insurance performs routine validations of the diagnoses by reviewing the original medical charts of all of the patients who applied for catastrophic illness registration. To comply with data privacy regulations, personal identities were encrypted and all data were analyzed in a de-identified manner
Study population
We investigated the database of NHIRD during year of 1995 to 2008. The index date for the study cohort was identified as the date of the first-time that had both a diagnosis of ESRD (ICD9-CM: 585.6, 585.9, 586) and a procedure of hemodialysis (ICD9-CM: 39.93) or peritoneal dialysis (ICD9-CM: 54.98). These ESRD patients have already undergone dialysis treatment without receiving renal transplantation. We identified all patients who were above 18 years old. The exclusion criteria included the following: (1) diagnosis of valvular heart disease (ICD9-CM code: 394.X-396.X, 398.X), (2) prior ambulatory visit for AF (ICD9-CM code, 427.3), (3) receiving beta-blockers less than 30 days. A flowchart for the identification of study subjects is shown in Fig. 1.
Drug use, covariates and outcomes
Patients were classified into use and non-use of beta-blockers. We had 12 kinds of beta-blockers (around 90 of generic drugs with different doses). To simplify the presentation, we used the total treatment duration of beta-blockers to demonstrate the dose-response effect. The majority of the treatment frequencies for beta-blockers were once daily (qd; 20 to 30%) and twice a day (bid; 70 to 80%).
Comorbidity was defined by diagnoses at hospital discharge or in clinic records. For our study population, we searched the database to see if they had HTN(ICD-9-CM codes: 401.X-405.X), DM (250.X, 249.X), dyslipidemia (272.X), ischemic stroke (ICD9-CM code, 434.X), hemorrhagic stroke (ICD9-CM code, 430.X), CAD (coronary artery disease, ICD9-CM code, 411.X-414.X, V17.3, V81.0), CHF hospitalization (ICD9-CM code, 428.0-428.3, 429.9), PAD (peripheral artery disease, ICD9-CM code, 250.7, 443.X, 444.2). Medications that were dispensed at time of index date, including beta-blockers, angiotensin converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), calcium channel blockers (CCBs), diuretics, statin, oral anti-diabetic drugs (OADs) and insulin were identified.
The main purpose of the study was to compare long-term development of AF among patients with and without taking beta-blockers. Diagnosis of AF was based on ICD-9-CM coding (ICD9-CM code, 427.3) in any ambulatory visit and discharge diagnoses.
Statistical analyses
For comparison of the baseline characteristics between two groups, Student’s t test was used for continuous variables and chi-square test was employed for categorical variables. Because patients were not randomly allocated to the two groups, we adjusted for age, gender, risk factors (HTN, DM and dyslipidemia), comorbidities (stroke/transient ischaemic accident, haemorrhagic stroke, CAD, PAD, CHF) and medication usage. In addition, the propensity score was applied to reduce the potential bias and to make the two groups more comparable. We derived a propensity score, which is the logit (probability) for receiving beta-blockers treatment from a logistic regression model by using all background covariates listed in Table 1 (age, gender, hemodialysis, HTN, DM, dyslipidemia, stroke, CAD, PAD, CHF hospitalization and medications). The predicted accuracy of the logistic model was assessed with an area under the receiver operating characteristic curve (C statistic), which was 0.864 (95% confidence interval [CI] 0.861–0.866). According to the propensity score, patients were selected by 1:1 matching without replacement using the nearest neighbor method. A caliper width of 0.15 standard deviations (SDs) was used for matching, because a width of between 0.05 and 0.30 SDs can eliminate almost 98% of the bias and correct the empirical type I error rates54. Baseline characteristics were compared within propensity score matched group.
For survival analyses, multivariate Cox’s proportional hazard regression analyses were used to derive the adjusted hazard ratios (HR) for developing AF by using patients without beta-blockers treatment as controls. Three models were used to adjust potential confounders. The model 1 was adjusted for age, gender, risk factors (HTN, DM and dyslipidemia), comorbidities (stroke/transient ischaemic accident, haemorrhagic stroke, CAD, PAD, CHF) and medication usage. The model 2 was similar to model 1 but included patients after propensity matching. In the model 3, the propensity score was entered as a covariate in the Cox’s proportional hazard regression model to adjust the potential selection bias. To test the consistency of the results, we also did subgroup analyses for different gender, age, presence of HTN, DM, cardiovascular disease (CVD) and CHF with adjustment for all confounders. The event-free survival curves of the two groups were illustrated by using the Kaplan-Meier method. The log-rank analysis was applied to test the differences in survival among groups.
All of the analyses were conducted using the Statistical Package for the Social Sciences (SPSS) for Windows, Version 19.0 (SPSS, Inc., Chicago, Illinois). A P value < 0.05 was considered statistically significant.
Additional Information
How to cite this article: Lin, T.-T. et al. Primary prevention of atrial fibrillation with beta-blockers in patients with end-stage renal disease undergoing dialysis. Sci. Rep. 5, 17731; doi: 10.1038/srep17731 (2015).
References
Rostock, T. et al. Atrial fibrillation begets atrial fibrillation in the pulmonary veins on the impact of atrial fibrillation on the electrophysiological properties of the pulmonary veins in humans. J Am Coll Cardiol. 51, 2153–2160. 2110.1016/j.jacc.2008.2102.2059. (2008).
Schotten, U., Verheule, S., Kirchhof, P. & Goette, A. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev. 91, 265–325. 210.1152/physrev.00031.02009. (2011).
Krahn, A. D., Manfreda, J., Tate, R. B., Mathewson, F. A. & Cuddy, T. E. The natural history of atrial fibrillation: incidence, risk factors and prognosis in the Manitoba Follow-Up Study. Am J Med. 98, 476–484 (1995).
Benjamin, E. J. et al. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 271, 840–844 (1994).
Alonso, A. et al. Chronic kidney disease is associated with the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 123, 2946–2953. 2910.1161/CIRCULATIONAHA.2111.020982. Epub 022011 Jun 020986. (2011).
Mathew, J. P. et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 291, 1720–1729 (2004).
Maisel, W. H., Rawn, J. D. & Stevenson, W. G. Atrial fibrillation after cardiac surgery. Ann Intern Med. 135, 1061–1073 (2001).
Ishii, Y. et al. Inflammation of atrium after cardiac surgery is associated with inhomogeneity of atrial conduction and atrial fibrillation. Circulation. 111, 2881–2888. Epub 2005 May 2831 (2005).
Villareal, R. P. et al. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J Am Coll Cardiol. 43, 742–748 (2004).
Mariscalco, G. et al. Atrial fibrillation after isolated coronary surgery affects late survival. Circulation. 118, 1612–1618. 1610.1161/CIRCULATIONAHA.1108.777789. Epub 772008 Sep 777729 (2008).
Arsenault, K. A. et al. Interventions for preventing post-operative atrial fibrillation in patients undergoing heart surgery. Cochrane Database Syst Rev. 1:CD003611., 10.1002/14651858.CD14003611.pub14651853 (2013).
Crystal, E., Connolly, S. J., Sleik, K., Ginger, T. J. & Yusuf, S. Interventions on prevention of postoperative atrial fibrillation in patients undergoing heart surgery: a meta-analysis. Circulation. 106, 75–80 (2002).
Eagle, K. A. et al. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery). J Am Coll Cardiol. 44, e213–310 (2004).
Tsikouris, J. P., Kluger, J., Song, J. & White, C. M. Changes in P-wave dispersion and P-wave duration after open heart surgery are associated with the peak incidence of atrial fibrillation. Heart Lung. 30, 466–471 (2001).
Franczyk-Skora, B. et al. Acute coronary syndromes in patients with chronic kidney disease. Curr Vasc Pharmacol. 11, 758–767 (2013).
Franczyk-Skora, B. et al. Prevention of sudden cardiac death in patients with chronic kidney disease. BMC Nephrol. 13, 162 10.1186/1471-2369-1113-1162. (2012).
Soliman, E. Z. et al. Chronic kidney disease and prevalent atrial fibrillation: the Chronic Renal Insufficiency Cohort (CRIC). Am Heart J. 159, 1102–1107. 1110.1016/j.ahj.2010.1103.1027. (2010).
Korantzopoulos, P. G. & Goudevenos, J. A. Atrial fibrillation in end-stage renal disease: an emerging problem. Kidney Int. 76, 247–249. 210.1038/ki.2009.1144. (2009).
Vaziri, S. M., Larson, M. G., Benjamin, E. J. & Levy, D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation. 89, 724–730 (1994).
Siragy, H. M. & Carey, R. M. Role of the intrarenal renin-angiotensin-aldosterone system in chronic kidney disease. Am J Nephrol 31, 541–550. 510.1159/000313363. Epub 000312010 May 000313318. (2010).
Aviles, R. J. et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 108, 3006–3010. Epub 2003 Nov 3017 (2003).
Olshansky, B. et al. The Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study: approaches to control rate in atrial fibrillation. J Am Coll Cardiol. 43, 1201–1208 (2004).
Camm, A. J. et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 31, 2369–2429. 2310.1093/eurheartj/ehq2278. Epub 2010 Aug 2329 (2010).
January, C. T. et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 64, e1–76. 10.1016/j.jacc.2014.1003.1022. Epub 2014 Mar 1028 (2014).
Camm, A. J. et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. European heart journal 33, 2719–2747, 10.1093/eurheartj/ehs253 (2012).
Khatib, R., Joseph, P., Briel, M., Yusuf, S. & Healey, J. Blockade of the renin-angiotensin-aldosterone system (RAAS) for primary prevention of non-valvular atrial fibrillation: a systematic review and meta analysis of randomized controlled trials. International journal of cardiology 165, 17–24, 10.1016/j.ijcard.2012.02.009 (2013).
Schmieder, R. E. et al. Reduced incidence of new-onset atrial fibrillation with angiotensin II receptor blockade: the VALUE trial. Journal of hypertension 26, 403–411, 10.1097/HJH.0b013e3282f35c67 (2008).
Yusuf, S. et al. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet (London, England) 372, 1174–1183, 10.1016/s0140-6736(08)61242-8 (2008).
Maggioni, A. P. et al. Valsartan reduces the incidence of atrial fibrillation in patients with heart failure: results from the Valsartan Heart Failure Trial (Val-HeFT). American heart journal 149, 548–557, 10.1016/j.ahj.2004.09.033 (2005).
US Renal Data System: USRDS 2005 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (2005).
Nimmo, C., Wright, M. & Goldsmith, D. Management of atrial fibrillation in chronic kidney disease: double trouble. American heart journal 166, 230–239, 10.1016/j.ahj.2013.05.010 (2013).
Zimmerman, D. et al. Systematic review and meta-analysis of incidence, prevalence and outcomes of atrial fibrillation in patients on dialysis. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association 27, 3816–3822, 10.1093/ndt/gfs416 (2012).
Himmelfarb, J., Stenvinkel, P., Ikizler, T. A. & Hakim, R. M. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney international 62, 1524–1538, 10.1046/j.1523-1755.2002.00600.x (2002).
Vaziri, N. D. Oxidative stress in uremia: nature, mechanisms and potential consequences. Seminars in nephrology 24, 469–473 (2004).
Franczyk-Skora, B., Gluba, A., Olszewski, R., Banach, M. & Rysz, J. Heart function disturbances in chronic kidney disease - echocardiographic indices. Arch Med Sci. 10, 1109–1116. 1110.5114/aoms.2014.47822. (2014).
Vazquez, E. et al. Comparison of prognostic value of atrial fibrillation versus sinus rhythm in patients on long-term hemodialysis. The American journal of cardiology 92, 868–871 (2003).
Wiesholzer, M. et al. Incidence of stroke among chronic hemodialysis patients with nonrheumatic atrial fibrillation. American journal of nephrology 21, 35–39, 46216 (2001).
Chan, K. E., Lazarus, J. M., Thadhani, R. & Hakim, R. M. Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. Journal of the American Society of Nephrology: JASN 20, 2223–2233, 10.1681/asn.2009030319 (2009).
Winkelmayer, W. C., Liu, J., Setoguchi, S. & Choudhry, N. K. Effectiveness and safety of warfarin initiation in older hemodialysis patients with incident atrial fibrillation. Clinical journal of the American Society of Nephrology: CJASN 6, 2662–2668, 10.2215/cjn.04550511 (2011).
Savelieva, I., Kakouros, N., Kourliouros, A. & Camm, A. J. Upstream therapies for management of atrial fibrillation: review of clinical evidence and implications for European Society of Cardiology guidelines. Part I: primary prevention. Europace. 13, 308–328. 310.1093/europace/eur1002. (2011).
Schneider, M. P. et al. Prevention of atrial fibrillation by Renin-Angiotensin system inhibition a meta-analysis. J Am Coll Cardiol. 55, 2299–2307. 2210.1016/j.jacc.2010.2201.2043. (2010).
Jibrini, M. B., Molnar, J. & Arora, R. R. Prevention of atrial fibrillation by way of abrogation of the renin-angiotensin system: a systematic review and meta-analysis. Am J Ther. 15, 36–43. 10.1097/MJT.1090b1013e31804beb31859. (2008).
Goette, A. et al. Angiotensin II-antagonist in paroxysmal atrial fibrillation (ANTIPAF) trial. Circulation. Arrhythmia and electrophysiology 5, 43–51, 10.1161/circep.111.965178 (2012).
Yamashita, T. et al. Randomized trial of angiotensin II-receptor blocker vs. dihydropiridine calcium channel blocker in the treatment of paroxysmal atrial fibrillation with hypertension (J-RHYTHM II study). Europace: European pacing, arrhythmias and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias and cardiac cellular electrophysiology of the European Society of Cardiology 13, 473–479, 10.1093/europace/euq439 (2011).
Yang, Q., Qi, X. & Li, Y. The preventive effect of atorvastatin on atrial fibrillation: a meta-analysis of randomized controlled trials. BMC cardiovascular disorders 14, 99, 10.1186/1471-2261-14-99 (2014).
Savelieva, I., Kakouros, N., Kourliouros, A. & Camm, A. J. Upstream therapies for management of atrial fibrillation: review of clinical evidence and implications for European Society of Cardiology guidelines. Part I: primary prevention. Europace: European pacing, arrhythmias and cardiac electrophysiology: journal of the working groups on cardiac pacing, arrhythmias and cardiac cellular electrophysiology of the European Society of Cardiology 13, 308–328, 10.1093/europace/eur002 (2011).
Dimmer, C. et al. Variations of autonomic tone preceding onset of atrial fibrillation after coronary artery bypass grafting. The American journal of cardiology 82, 22–25 (1998).
Sharifov, O. F. et al. Roles of adrenergic and cholinergic stimulation in spontaneous atrial fibrillation in dogs. Journal of the American College of Cardiology 43, 483–490, 10.1016/j.jacc.2003.09.030 (2004).
Sezai, A. et al. Feasibility of landiolol and bisoprolol for prevention of atrial fibrillation after coronary artery bypass grafting: a pilot study. The Journal of thoracic and cardiovascular surgery 144, 1241–1248, 10.1016/j.jtcvs.2012.06.042 (2012).
Ye, S., Zhong, H., Yanamadala, S. & Campese, V. M. Oxidative stress mediates the stimulation of sympathetic nerve activity in the phenol renal injury model of hypertension. Hypertension 48, 309–315, 10.1161/01.HYP.0000231307.69761.2e (2006).
Campese, V. M. & Krol, E. Neurogenic factors in renal hypertension. Current hypertension reports 4, 256–260 (2002).
Nangaku, M. & Fujita, T. Activation of the renin-angiotensin system and chronic hypoxia of the kidney. Hypertens Res. 31, 175–184. 110.1291/hypres.1231.1175. (2008).
Lin, T. T. et al. Primary prevention of atrial fibrillation with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in patients with end-stage renal disease undergoing dialysis. Kidney international 88, 378–385, 10.1038/ki.2015.96 (2015).
Austin, P. C. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 10, 150–161. 110.1002/pst.1433. (2011).
Acknowledgements
This study was based in part on data from the National Health Insurance Research Database provided by the National Health Insurance Administration, Ministry of Health and Welfare, Executive Yuan, Taiwan and managed by National Health Research Institutes, Taiwan. All the data used in our study were released and approved by the Collaboration Center of Health Information Application, Ministry of Health and Welfare, Executive Yuan, Taiwan. The interpretations and conclusions contained herein do not represent those of the National Health Insurance Administration, Ministry of Health and Welfare, Executive Yuan, Taiwan or National Health Research Institutes, Taiwan.
Author information
Authors and Affiliations
Contributions
The specific contributions made to this article by the listed authors are as follows: T.-T.L. and J.-Y.C. drafted the manuscript and plotted the figures. M.-T.L. performed data analysis. C.-T.T., J.-J.H., F.-T.C. and J.-L.L. designed the study. L.-Y.L. revised the manuscript and took responsibility for the interpretation of the results. All authors have reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Lin, TT., Chiang, JY., Liao, MT. et al. Primary prevention of atrial fibrillation with beta-blockers in patients with end-stage renal disease undergoing dialysis. Sci Rep 5, 17731 (2016). https://doi.org/10.1038/srep17731
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep17731
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.