Abstract
Techniques for rapid design of dielectric materials with appropriate permittivity for many important technological applications are urgently needed. It is found that functional structure blocks (FSBs) are helpful in rational design of inorganic dielectrics with expected permittivity. To achieve this, coordination polyhedra are parameterized as FSBs and a simple empirical model to evaluate permittivity based on these FSB parameters is proposed. Using this model, a wide range of examples including ferroelectric, high/low permittivity materials are discussed, resulting in several candidate materials for experimental follow-up.
Similar content being viewed by others
Introduction
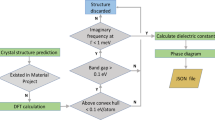
Dielectric materials are essential for many technological applications in optical, electronic and micro-electronic devices. For instance, high-permittivity materials are required for gate dielectrics and high-energy storage capacitors and low-permittivity dielectrics are necessary for transparent windows and miniaturized integrated circuits. The search for these dielectric materials over a wide range of compounds is time-consuming. A major reason is the lack of a clear and intuitive data set to give an idea about which materials should be focused on1. Fortunately, we now have computational tools such as codes based on density functional theory2,3 (DFT), capable of accurately predicting many important materials properties. With the help of computations, materials discovery can be accelerated4,5,6.
Up to now, high-throughput computational approach have been employed to screen thousands of compounds for new materials7,8,9,10,11,12,13,14. Structure prediction methods15, such as USPEX16,17, have also been developed to optimize certain properties of materials with only the chemical composition given18,19,20,21. However, the efficiency of these theoretical methods requires a fast and accurate evaluation of the properties of interest, while dielectric properties are relatively time-consuming. Therefore, it would be desirable to find a way to compute them from crystal structure, most transparently using functional structure blocks (FSBs), which are directly linked to the materials properties. The application of this FSB method mainly depends on: (1) the determination of a suitable FSB for a certain property of materials; and (2) the establishment of an explicit relationship between this property and its FSB. With such structure-property relations, one can quantitatively or qualitatively evaluate properties for a material in seconds. In this paper, we will demonstrate that the idea of FSBs could be very useful for rational design of materials with expected permittivity.
Inspired by Rignanese et al.22 and our previous studies21,23, we choose the coordination polyhedron as FSB for permittivity due to its major and easy to rationalize effect on permittivities of materials. Coordination polyhedron to a very large extent determines many aspects of lattice dynamics and thus can be used to determine permittivity21,22. Rignanese et al.22 proposed an empirical model to calculate permittivity, for each coordination polyhedron using three characteristic parameters (electronic polarizability  , charge
, charge  and force constant
and force constant  ). In this present study, we suggest a simplified empirical model with each type of coordination polyhedra characterized by two parameters: electronic polarizability
). In this present study, we suggest a simplified empirical model with each type of coordination polyhedra characterized by two parameters: electronic polarizability  and ionic oscillator strength
and ionic oscillator strength  . Furthermore, by introducing the volume
. Furthermore, by introducing the volume  of each type of polyhedron, we can extend our model to estimate permittivity of a crystal structure provided that the type of coordination polyhedron is known. This means that dielectric materials with expected permittivity could be constructed by selecting appropriate coordination polyhedra.
of each type of polyhedron, we can extend our model to estimate permittivity of a crystal structure provided that the type of coordination polyhedron is known. This means that dielectric materials with expected permittivity could be constructed by selecting appropriate coordination polyhedra.
Results and Discussions
Description of the model
According to Rignanese’s model22, it is possible to evaluate the electronic24, lattice and static permittivities of a given structure based on its electronic polarizability  , charge
, charge  and force constant
and force constant  :
:


where  is the electronic permittivity;
is the electronic permittivity;  is the lattice permittivity;
is the lattice permittivity;  is the static permittivity; and
is the static permittivity; and  is the volume of the structure. They define
is the volume of the structure. They define  ,
,  and
and  values for each type of coordination polyhedron i and assuming that:
values for each type of coordination polyhedron i and assuming that:

where  is the number of type-i coordination polyhedron contained in a structure. Summation is done over all types of coordination polyhedra. The optimal
is the number of type-i coordination polyhedron contained in a structure. Summation is done over all types of coordination polyhedra. The optimal  ,
,  and
and  values for each type of coordination polyhedron i can be determined using least-squares method based on the
values for each type of coordination polyhedron i can be determined using least-squares method based on the  ,
,  and
and  values calculated from first principles for a set of materials. However,
values calculated from first principles for a set of materials. However,  obtained by their model is sometimes very different from that calculated from first principles. This may be due to the fact that
obtained by their model is sometimes very different from that calculated from first principles. This may be due to the fact that  and
and  are considered as two independent variables in their model, which, however, may be correlated to each other. Therefore, we suggest defining a single parameter of ionic oscillator strength
are considered as two independent variables in their model, which, however, may be correlated to each other. Therefore, we suggest defining a single parameter of ionic oscillator strength  :
:

Then, the lattice permittivity  can be calculated as:
can be calculated as:

By analogy with  , we define
, we define  for each type of coordination polyhedron i such that:
for each type of coordination polyhedron i such that:

The optimal values  can be determined in the same way as for
can be determined in the same way as for  . As shown in the following part of this paper,
. As shown in the following part of this paper,  obtained from our simplified model improve upon those calculated from Rignanese’s model in most cases.
obtained from our simplified model improve upon those calculated from Rignanese’s model in most cases.
Test of the model
We have calculated permittivity of various inorganic compounds constructed from three binary oxide systems (MgO, Al2O3 and SiO2). With the crystal structures of these compounds obtained from Materials Project1, we performed full structure relaxation before calculating permittivity using the density functional perturbation theory (DFPT25) approach. Structural information and DFPT permittivities of these compounds can be found as Supplementary Table Is. The optimal  and
and  values of seven coordination polyhedra, MgO4, MgO6, AlO4, AlO5, AlO6, SiO4 and SiO6 obtained in our model are listed in Table 1.
values of seven coordination polyhedra, MgO4, MgO6, AlO4, AlO5, AlO6, SiO4 and SiO6 obtained in our model are listed in Table 1.
 in Å3), ionic oscillator strengths (
in Å3), ionic oscillator strengths ( in Å3), effective volumes (
in Å3), effective volumes ( in Å3), electronic polarizabilities per volume (
in Å3), electronic polarizabilities per volume ( ) and ionic oscillator strengths per volume (
) and ionic oscillator strengths per volume ( ) of 26 coordination polyhedra.
) of 26 coordination polyhedra.In Fig. 1,  and
and  values of MgO, Al2O3 and SiO2 compounds given by our model are compared to those calculated from DFPT approach, with quite good agreement for most of the structures. In particular,
values of MgO, Al2O3 and SiO2 compounds given by our model are compared to those calculated from DFPT approach, with quite good agreement for most of the structures. In particular,  values obtained in our model agree very well with those computed by the DFPT approach, with an average relative error as low as 1.5%. Although a few
values obtained in our model agree very well with those computed by the DFPT approach, with an average relative error as low as 1.5%. Although a few  values have error higher than 10%, it can be concluded that our
values have error higher than 10%, it can be concluded that our  values of MgO4, MgO6, AlO4, AlO5, AlO6, SiO4 and SiO6 coordination polyhedra are reliable.
values of MgO4, MgO6, AlO4, AlO5, AlO6, SiO4 and SiO6 coordination polyhedra are reliable.
To test the applicability of our model, we evaluated permittivities of many ternary and quaternary oxides in (MgO)x(Al2O3)y(SiO2)z system (see Table 2). DFPT results obtained by us and some experimentally or theoretically reported values are also listed in Table 2 for comparison. We can see that our model with optimized  and
and  is really helpful to evaluate materials permittivity. Moreover, our model may provide a way to obtain permittivity for very complex systems where DFPT approach is not feasible, e.g., enstatite MgSiO3 (80 atoms/cell) listed in Table 2.
is really helpful to evaluate materials permittivity. Moreover, our model may provide a way to obtain permittivity for very complex systems where DFPT approach is not feasible, e.g., enstatite MgSiO3 (80 atoms/cell) listed in Table 2.
 and static−
and static− ) of some ternary and quaternary oxides in the (MgO)x(Al2O3)y(SiO2)z system.
) of some ternary and quaternary oxides in the (MgO)x(Al2O3)y(SiO2)z system.However, one must keep in mind the limitations of the model (see  values shown in Fig. 1). We conclude that our simplified model is not suitable for materials with low-frequency polar modes having large contributions (due to large
values shown in Fig. 1). We conclude that our simplified model is not suitable for materials with low-frequency polar modes having large contributions (due to large  values) to the lattice permittivity. We return to this point later in this paper.
values) to the lattice permittivity. We return to this point later in this paper.
Our model can also be extended to evaluate permittivity of a hypothetical structure, for which only the types of coordination polyhedra are given. To achieve this, we define volume  for each type of coordination polyhedron i and determine optimal
for each type of coordination polyhedron i and determine optimal  values in the same way as for
values in the same way as for  and
and  (as listed in Table 1). The addition of
(as listed in Table 1). The addition of  of coordination polyhedron i can reproduce volume of a structure well (as shown in Fig. 2). Then the
of coordination polyhedron i can reproduce volume of a structure well (as shown in Fig. 2). Then the  (
( ) values of a structure can be obtained from:
) values of a structure can be obtained from:

The corresponding  (
( ) values are comparable to those calculated from DFPT approach (see Fig. 3). In this way, permittivity of a hypothetical structure can be reasonably evaluated.
) values are comparable to those calculated from DFPT approach (see Fig. 3). In this way, permittivity of a hypothetical structure can be reasonably evaluated.
Application of the model
The  ,
,  and
and  values of each type of coordination polyhedra obtained from our model are helpful to design dielectric materials with expected permittivity. First, we extended our model to study some other oxides, nitrides and fluorides (see Supplementary Table IIs). We obtained
values of each type of coordination polyhedra obtained from our model are helpful to design dielectric materials with expected permittivity. First, we extended our model to study some other oxides, nitrides and fluorides (see Supplementary Table IIs). We obtained  ,
,  and
and  values for another 19 coordination polyhedra (see Table 1). With the
values for another 19 coordination polyhedra (see Table 1). With the  ,
,  and
and  values of 26 coordination polyhedra listed in Table 1, we illustrated how to rationally design ferroelectric and high/low permittivity materials.
values of 26 coordination polyhedra listed in Table 1, we illustrated how to rationally design ferroelectric and high/low permittivity materials.
We have calculated  of 95 compounds using
of 95 compounds using  values of these 26 coordination polyhedra. Some of these compounds are listed in Table 2. The complete list of compounds can be found as Supplementary Tables Is and IIs. We compare
values of these 26 coordination polyhedra. Some of these compounds are listed in Table 2. The complete list of compounds can be found as Supplementary Tables Is and IIs. We compare  values of these 95 compounds with those calculated from DFPT approach (see Fig. 4). The agreement between the two data sets is good. However, there are two deviating structures, P42/nmc HfO2 and Pbnm MgSiO3, for which the actual
values of these 95 compounds with those calculated from DFPT approach (see Fig. 4). The agreement between the two data sets is good. However, there are two deviating structures, P42/nmc HfO2 and Pbnm MgSiO3, for which the actual  is much higher than that from our model. We found that the “unusual” enhancement of
is much higher than that from our model. We found that the “unusual” enhancement of  is related to large
is related to large  values. This may originate from low-frequency polar phonon modes, which means that these two structures can be close to a ferroelectric instability.
values. This may originate from low-frequency polar phonon modes, which means that these two structures can be close to a ferroelectric instability.
In fact, the P42/nmc HfO2 is a well-known ferroelectric material. Another structure, Pbnm MgSiO3, possesses a perovskite structure adopted by many ferroelectric materials. We calculated the contributions to  from each polar phonon mode of Pbnm MgSiO3 (as listed Table 3). The Pbnm MgSiO3 indeed possesses a low-frequency polar phonon mode (at 175 cm−1) contributing to
from each polar phonon mode of Pbnm MgSiO3 (as listed Table 3). The Pbnm MgSiO3 indeed possesses a low-frequency polar phonon mode (at 175 cm−1) contributing to  much more than other phonon modes. In other words, our model underestimates permittivities of ferroelectrics and crystals with softened polar modes. This can actually be used for rapid screening of potential ferroelectric materials.
much more than other phonon modes. In other words, our model underestimates permittivities of ferroelectrics and crystals with softened polar modes. This can actually be used for rapid screening of potential ferroelectric materials.
 [cm−1]) and their contributions to the permittivity (
[cm−1]) and their contributions to the permittivity ( ) computed for Pbnm MgSiO346.
) computed for Pbnm MgSiO346.Our model is also helpful in the design of materials with high/low permittivity. Our results show, quite intuitively, that coordination polyhedra with high  (
( ) and low
) and low  are favorable for high dielectric permittivity.
are favorable for high dielectric permittivity.
At a glance at Table I, we can find that HfO8 has much higher  and
and  values than others among the 26 coordination polyhedra. Indeed, Hf oxides are excellent high-permittivity oxides (ref. 20). On the other hand, SiO4 tetrahedron possesses the lowest
values than others among the 26 coordination polyhedra. Indeed, Hf oxides are excellent high-permittivity oxides (ref. 20). On the other hand, SiO4 tetrahedron possesses the lowest  and
and  values among O-based coordination polyhedra. Indeed, SiO2 (quartz and silica glass) with SiO4 tetrahedra is a well-known low-permittivity material in micro-electronics industry.
values among O-based coordination polyhedra. Indeed, SiO2 (quartz and silica glass) with SiO4 tetrahedra is a well-known low-permittivity material in micro-electronics industry.
Noticeably,  and
and  values of N-based coordination polyhedra are higher than those of O-based coordination polyhedra. For instance, AlN6 coordination polyhedron has much higher
values of N-based coordination polyhedra are higher than those of O-based coordination polyhedra. For instance, AlN6 coordination polyhedron has much higher  and
and  values than AlO6. We may expect high-permittivity in nitrides, e.g., Hf3N4 with HfN8 coordination polyhedron. As listed in Table 1,
values than AlO6. We may expect high-permittivity in nitrides, e.g., Hf3N4 with HfN8 coordination polyhedron. As listed in Table 1,  and
and  values of HfN8 coordination polyhedron are higher than those of the HfO8 polyhedron. Therefore,
values of HfN8 coordination polyhedron are higher than those of the HfO8 polyhedron. Therefore,  Hf3N4 with HfN8 coordination polyhedron has higher permittivities than most of hafnium oxides (see Supplementary Table IIs).
Hf3N4 with HfN8 coordination polyhedron has higher permittivities than most of hafnium oxides (see Supplementary Table IIs).
For the design of low-permittivity materials, we can immediately expect that permittivity of an oxide can be decreased by replacing O with F (see Table 1). Experimentally, SiF4 material with SiF4 tetrahedra has much lower permittivity than quartz26,27. In a similar way, we can expect that  and
and  values of MgF4 coordination polyhedron may be much lower than those of MgO4 polyhedron. Therefore, we try to design low-permittivity MgF2 material with MgF4 coordination polyhedron. We constructed a new
values of MgF4 coordination polyhedron may be much lower than those of MgO4 polyhedron. Therefore, we try to design low-permittivity MgF2 material with MgF4 coordination polyhedron. We constructed a new  MgF2 phase (Fig. 5(a)) with very low permittivity using
MgF2 phase (Fig. 5(a)) with very low permittivity using  SiO2 structure (cristobalite) with SiO4 tetrahedra (detailed structural information can be found as Supplementary Table IIIs). The static permittivity
SiO2 structure (cristobalite) with SiO4 tetrahedra (detailed structural information can be found as Supplementary Table IIIs). The static permittivity  of
of  MgF2 (2.5) is much lower than that of quartz (3.927) and comparable to most low-permittivity polymers. The dynamical and mechanical stability of
MgF2 (2.5) is much lower than that of quartz (3.927) and comparable to most low-permittivity polymers. The dynamical and mechanical stability of  MgF2 was verified by phonon and elastic constants calculations (see Supplementary Fig. 1s and Table IVs). The enthalpy of
MgF2 was verified by phonon and elastic constants calculations (see Supplementary Fig. 1s and Table IVs). The enthalpy of  MgF2 phase is only 0.1 eV/atom higher than that of the most stable MgF2 structure (P42/mnm phase). Moreover, this inorganic material may have a better mechanical strength than polymers (see Supplementary Table IVs). This suggests that
MgF2 phase is only 0.1 eV/atom higher than that of the most stable MgF2 structure (P42/mnm phase). Moreover, this inorganic material may have a better mechanical strength than polymers (see Supplementary Table IVs). This suggests that  MgF2 may be synthesized and tested as a potential low-permittivity material.
MgF2 may be synthesized and tested as a potential low-permittivity material.
From the Materials Project, we also found a near-ground-state BeF2 structure ( ) with BeF4 coordination polyhedra, as shown in Fig. 5(b). The static permittivity
) with BeF4 coordination polyhedra, as shown in Fig. 5(b). The static permittivity  of
of  BeF2 is 2.5, indicating that BeF2 is also a good low-permittivity material. We suggest that compounds constructed from LiF4, BF4, NaF4 and AlF4 coordination polyhedra may also have low permittivities, e.g.,
BeF2 is 2.5, indicating that BeF2 is also a good low-permittivity material. We suggest that compounds constructed from LiF4, BF4, NaF4 and AlF4 coordination polyhedra may also have low permittivities, e.g.,  of P3121 LiBF4 with LiF4 and BF4 coordination polyhedra can be as low as 3.6.
of P3121 LiBF4 with LiF4 and BF4 coordination polyhedra can be as low as 3.6.
We have to mention that coordination number is an important factor to design high/low-permittivity materials. There is a trend21,23: low coordination number, low permittivity. Our present study agrees with this trend well; coordination polyhedra with low coordination number have low  and
and  values. For example, our study shows that the
values. For example, our study shows that the  SiC2N4 structure, with 1/3 SiN4 and 2/3 CN2 coordination polyhedra, has much lower permittivity (4.6) than P63/m Si3N4 (8.3) containing SiN4 coordination polyhedra.
SiC2N4 structure, with 1/3 SiN4 and 2/3 CN2 coordination polyhedra, has much lower permittivity (4.6) than P63/m Si3N4 (8.3) containing SiN4 coordination polyhedra.
To summarize, we have presented a method for designing new inorganic dielectrics with expected permittivity is discussed. Coordination polyhedron is adopted as the functional structural block (FSB) of permittivity. Three parameters (electronic polarizability  , ionic oscillator strength
, ionic oscillator strength  and volume
and volume  ) are chosen to characterize each coordination polyhedron. We show applications of this model evaluate materials permittivity. Results derived from this model agree well with those from density-functional perturbation theory. Moreover,
) are chosen to characterize each coordination polyhedron. We show applications of this model evaluate materials permittivity. Results derived from this model agree well with those from density-functional perturbation theory. Moreover,  ,
,  and
and  values assigned to coordination polyhedra may be helpful to make intuitive choices of materials to focus on. Successful applications include ferroelectric, high- and low-permittivity materials.
values assigned to coordination polyhedra may be helpful to make intuitive choices of materials to focus on. Successful applications include ferroelectric, high- and low-permittivity materials.
Methods
Before calculating the properties, we perform full structure relaxation using density functional theory (DFT2,3) as implemented in the Vienna ab intio Simulation Package (VASP28) with the PBEsol-GGA29,30 exchange-correlation functional. The all-electron projector-augmented wave (PAW) method31 is used, with a plane-wave energy cutoff of 900 eV and k-point meshes with reciprocal-space resolution of  . These settings enable excellent convergence for the energy differences, stress tensors and structural parameters. With fully relaxed structures, dielectric25 and mechanical32 properties (e.g. the elastic constants) were computed. Permittivities and phonon dispersion curves are calculated using density functional perturbation theory (DFPT25). Phonon dispersion curves were obtained by PHONOPY33.
. These settings enable excellent convergence for the energy differences, stress tensors and structural parameters. With fully relaxed structures, dielectric25 and mechanical32 properties (e.g. the elastic constants) were computed. Permittivities and phonon dispersion curves are calculated using density functional perturbation theory (DFPT25). Phonon dispersion curves were obtained by PHONOPY33.
Additional Information
How to cite this article: Xie, C. et al. Rational design of inorganic dielectric materials with expected permittivity. Sci. Rep. 5, 16769; doi: 10.1038/srep16769 (2015).
References
Jain, A. et al. Commentary: The materials project: A materials genome approach to accelerating materials innovation. APL Materials 1, 011002 (2013).
Hohenberg, P. & Kohn, W. Inhomogeneous electron gas. Phys. Rev. 136, B864–B871 (1964).
Kohn,W. & Sham, L. J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 140, A1133–A1138 (1965).
Ma, R. et al. Rationally designed polyimides for high-energy density capacitor applications. ACS Appl. Mat. Interfaces 6, 10445–10451 (2014).
Sharma, V. et al. Rational design of all organic polymer dielectrics. Nature Commun. 5, 4845 (2014).
Duan, D. et al. Pressure-induced metallization of dense (H2S)2H2 with high-Tc superconductivity. Sci. Rep. 4, 6968 (2014).
Yu, L. & Zunger, A. Identification of potential photovoltaic absorbers based on first-principles spectroscopic screening of materials. Phys. Rev. Lett. 108, 068701 (2012).
Yang, K., Setyawan, W., Wang, S., Nardelli, M. B. & Curtarolo, S. A search model for topological insulators with high-throughput robustness descriptors. Nature Mater. 11, 614–619 (2012).
Lin, L.-C. et al. In silico screening of carbon-capture materials. Nature Mater. 11, 633–641 (2012).
Greeley, J., Jaramillo, T. F., Bonde, J., Chorkendorff, I. & Nørskov, J. K. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nature Mater. 5, 909–913 (2006).
Wang, S., Wang, Z., Setyawan, W., Mingo, N. & Curtarolo, S. Assessing the thermoelectric properties of sintered compounds via high-throughput Ab-Initio calculations. Phys. Rev. X 1, 021012 (2011).
Armiento, R., Kozinsky, B., Fornari, M. & Ceder, G. Screening for high-performance piezoelectrics using high-throughput density functional theory. Phys. Rev. B 84, 014103 (2011).
Lu, J., Fang, Z. Z., Choi, Y. J. & Sohn, H. Y. Potential of binary lithium magnesium nitride for hydrogen storage applications. J. Phys. Chem. C 111, 12129–12134 (2007).
Jain, A. et al. A computational investigation of Li9M3(P2O7)3(PO4)2(M = V, Mo) as cathodes for Li ion batteries. J. Electr. Soc. 159, A622–A633 (2012).
Oganov, A. R. Modern methods of crystal structure prediction (John Wiley & Sons, 2011).
Oganov, A. R. & Glass, C. W. Crystal structure prediction using ab initio evolutionary techniques: Principles and applications. J. Chem. Phys. 124, 244704 (2006).
Glass, C. W., Oganov, A. R. & Hansen, N. Uspex-evolutionary crystal structure prediction. Comput. Phys. Commun. 175, 713–720 (2006).
Lyakhov, A. O. & Oganov, A. R. Evolutionary search for superhard materials: Methodology and applications to forms of carbon and TiO2 . Phys. Rev. B 84, 092103 (2011).
Zhu, Q., Oganov, A. R., Salvadó, M. A., Pertierra, P. & Lyakhov, A. O. Denser than diamond: Ab initio search for superdense carbon allotropes. Phys. Rev. B 83, 193410 (2011).
Zeng, Q. et al. Evolutionary search for new high-k dielectric materials: methodology and applications to hafnia-based oxides. Acta Cryst. C70, 76–84 (2014).
Xie, C., Zeng, Q., Oganov, A. R. & Dong, D. Discovering low-permittivity materials: Evolutionary search for MgAl2O4 polymorphs. Appl. Phys. Lett. 105, 022907 (2014).
Rignanese, G.-M., Detraux, F., Gonze, X., Bongiorno, A. & Pasquarello, A. Dielectric constants of Zr silicates: A first-principles study. Phys. Rev. Lett. 89, 117601 (2002).
Xie, C. et al. First-principles calculations of the dielectric and vibrational properties of ferroelectric and paraelectric BaAl2O4 . Phys. Lett. A 378, 1867–1870 (2014).
Blythe, A. R. & Bloor, D. Electrical properties of polymers (Cambridge University Press, 2005).
Baroni, S., de Gironcoli, S., Dal Corso, A. & Giannozzi, P. Phonons and related crystal properties from density-functional perturbation theory. Rev. Mod. Phys. 73, 515–562 (2001).
Rado, W. G. The nonlinear third order dielectric susceptibility coefficients of gases and optical third harmonic generation. Appl. Phys. Lett. 11, 123–125 (1967).
Robertson, J. High dielectric constant gate oxides for metal oxide Si transistors. Rep. Prog. Phys. 69, 327 (2006).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P. et al. Restoring the density-gradient expansion for exchange in solids and surfaces. Phys. Rev. Lett. 100, 136406 (2008).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Cowley, R. A. Acoustic phonon instabilities and structural phase transitions. Phys. Rev. B 13, 4877–4885 (1976).
Togo, A., Oba, F. & Tanaka, I. First-principles calculations of the ferroelastic transition between rutile-type and CaCl2-type SiO2 at high pressures. Phys. Rev. B 78, 134106 (2008).
Boguslavska, N. N., Venger, E. F., Vernidub, N. M., Pasechnik, Y. A. & Shportko, K. V. Reststrahlen spectroscopy of MgAl2O4 spinel. Semicond. Phys. Quantum Electron. Optoelectron. 5, 95–100 (2002).
Wang, C. C. & Zanzucchi, P. J. Dielectric and optical properties of stoichiometric magnesium aluminate spinel single crystals. J. Electrochem. Soc. 118, 586–591 (1971).
Surendran, K. P., Bijumon, P. V., Mohanan, P. & Sebastian, M. T. (1 − x)MgAl2O4−xTiO2 dielectrics for microwave and millimeter wave applications. Appl. Phys. A 81, 823–826 (2005).
Rosenholtz, J. L. & Smith, D. T. The dielectric constant of mineral powders (Rensselaer Polytechnic Institute, 1936).
Song, M. E. et al. Synthesis and microwave dielectric properties of MgSiO3 ceramics. J. Am. Ceram. Soc. 91, 2747–2750 (2008).
De La Pierre, M. et al. Performance of six functionals (LDA, PBE, PBESOL, B3LYP, PBE0 and WCLLYP) in the simulation of vibrational and dielectric properties of crystalline compounds. the case of forsterite MgSi2O4 . J. Comput. Chem. 32, 1775–1784 (2011).
Ohsato, H., Tsunooka, T., Sugiyama, T., Kakimoto, K.-I. & Ogawa, H. Forsterite ceramics for millimeterwave dielectrics. J. Electroceram. 17, 445–450 (2006).
Shannon, R. D. Dielectric polarizabilities of ions in oxides and fluorides. J. Appl. Phys. 73, 348–366 (1993).
Aryal, S., Rulis, P. & Ching, W. Y. Density functional calculations of the electronic structure and optical properties of aluminosilicate polymorphs (Al2SiO5). Am. Miner. 93, 114–123 (2008).
Eru, I., Peskovatskiy, S. & Chernets, A. Trivalent-iron-doped andalusite as crystal for microwave waves. IEEE J. Quantum Electron. 4, 723–727 (1968).
Mugeraya, S. & Prabhakar, B. R. Measurement of resistivity and dielectric constant of beach-sand minerals. J. Electrostat. 18, 109–112 (1986).
Sei, T., Eto, K. & Tsuchiya, T. The role of boron in low-temperature synthesis of indialite (α-Mg2Al4Si5O18) by sol–gel process. J. Mater. Sci. 32, 3013–3019 (1997).
Ohsato, H. et al. Sintering conditions of cordierite for microwave/millimeterwave dielectrics. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 55, 1081–1085 (2008).
Acknowledgements
This work was supported by the Foreign Talents Introduction and Academic Exchange Program of China (No. B08040), the National Science Foundation (EAR-1114313, DMR-1231586), DARPA (Grant No. W31P4Q1210008) and the Government of Russian Federation (grant 14.A21.31.0003). The authors also acknowledge the High Performance Computing Center of NWPU for the allocation of computing time on their machines.
Author information
Authors and Affiliations
Contributions
C.-W.X. and A.R.O. designed the project and performed the calculations. C.-W.X., A.R.O. and D.D. analyzed the data and wrote the paper. N.L. got the structural information of the structures studied in this paper. D.L. and T.T.D. helped to plot the figures.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Xie, C., Oganov, A., Dong, D. et al. Rational design of inorganic dielectric materials with expected permittivity. Sci Rep 5, 16769 (2015). https://doi.org/10.1038/srep16769
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep16769
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.







 MgF2 constructed from MgF4 coordination polyhedra; (b)
MgF2 constructed from MgF4 coordination polyhedra; (b)  BeF2 constructed from BeF4 coordination polyhedra. Blue spheres denote F atoms, brown spheres denote Mg atoms and green spheres denote Be atoms.
BeF2 constructed from BeF4 coordination polyhedra. Blue spheres denote F atoms, brown spheres denote Mg atoms and green spheres denote Be atoms.