Abstract
Red leaf color is an attractive trait of Malus families, including crabapple (Malus spp.); however, little is known about the molecular mechanisms that regulate the coloration. Dihydroflavonols are intermediates in the production of both colored anthocyanins and colorless flavonols and this current study focused on the gene expression balance involved in the relative accumulation of these compounds in crabapple leaves. Levels of anthocyanins and the transcript abundances of the anthocyanin biosynthetic gene, dihydroflavonol 4-reductase (McDFR) and the flavonol biosynthetic gene, flavonol synthase (McFLS), were assessed during the leaf development in two crabapple cultivars, ‘Royalty’ and ‘Flame’. The concentrations of anthocyanins and flavonols correlated with leaf color and we propose that the expression of McDFR and McFLS influences their accumulation. Further studies showed that overexpression of McDFR, or silencing of McFLS, increased anthocyanin production, resulting in red-leaf and red fruit peel phenotypes. Conversely, elevated flavonol production and green phenotypes in crabapple leaves and apple peel were observed when McFLS was overexpressed or McDFR was silenced. These results suggest that the relative activities of McDFR and McFLS are important determinants of the red color of crabapple leaves, via the regulation of the metabolic fate of substrates that these enzymes have in common.
Similar content being viewed by others
Introduction
Flavonoids are a class of plant secondary metabolites that collectively have diverse functions, including providing protection against abiotic stresses, particularly UV-irradiation and biotic factors, such as phytophagous insects, as well as attracting pollinators1,2. They also have considerable value as components of the human diet3 and are used in the pharmaceutical industry since they have antioxidative, anticancer and anti-inflammatory properties4,5. Flavonoids have been extensively studied in a range of land plants and have been divided into nine structural subclasses: chalcones, flavones, flavonols, dihydroflavonols, flavandiols, anthocyanins, proanthocyanidins, flavonoid isoflavonoids and aurones5. Moreover, many genes involved in the flavonoid biosynthetic pathway have been identified and characterized. For example, the genes encoding chalcone synthase (CHS), chalcone isomerase (CHI), F3H flavanone 3-hydroxylase (F3H), flavonoid 3′-monooxygenase (F3′H), dihydroflavonol 4-reductase (DFR), anthocyanidin synthase (ANS), uridine diphosphate glucose-flavonoid 3-O-glucosyl transferase (UFGT), flavonol synthase (FLS), leucoanthocyanidin reductase (LAR) and anthocyanidin reductase (ANR) have been characterized in several plant species6,7,8,9,10,11,12,13,14.
The flavonoid biosynthetic pathway lies downstream of the phenylpropanoid pathway and leads to the formation of anthocyanins and flavonols (Fig. 1). Chalcone synthase (CHS; EC 2.3.1.74) uses beta-coumaroyl-CoA and 3 Malonyl-CoA as substrates to form naringenin chalcone15. This condensation reaction is a key step in the pathway leading to the formation of flavonoids16. Next, the F3H converts naringenin to dihydrokaempferol. Then dihydrokaempferol and dihydroquercetin are converted to kaempferol and quercetin by FLS, respectively. DFR and FLS also catalyze competing reactions to generate products leading to the spectrum of downstream anthocyanins and flavonols17,18.
Diagrammatic representation of the flavonoid biosynthetic pathway in plant.
Enzymes required for flavonol and anthocyanin synthesis: CHS, chalcone synthase; CHI, chalcone isomerase; F3H, flavanone 3-hydroxylase; FLS, flavonol synthase; DFR, dihydroflavonol 4-reductase; F’3H, flavonoid 3′-monooxygenase; ANS, anthocyanidin synthase; GTs, glucosyltransferases; ATs, anthocyanin aromatic acyltransferases.
MYB family transcriptional factors are known to be involved in regulating the expression expression of flavonoid biosynthesis genes19. For example, in Arabidopsis thaliana, AtMYBL2 interacts with TT8 (TRANSPARENT TESTA 8) to reduce anthocyanin biosynthesis20. In the context of flavonoid biosynthesis, AtMYB12 is thought to effect flavonol production19,21 by regulation CHS, CHI, F3H and FLS1 gene expression levels22. In addition, the expression of three allelic apple (Malus × domestic) genes (MYB10, MYB1 and MYBA) was shown to strongly correlate with the accumulation of anthocyanins in fruit23,24,25.
The pigmentation of many plant organs results from the presence of some of these flavonoid classes and, in many instances, a primary determinant of color is the accumulation of anthocyanins. The synthesis of anthocyanins occurs via a key branch in the flavonoid biosynthesis pathway, involving the action of the enzyme DFR on the dihydroflavonols dihydroquercetin, dihydrokaempferol or dihydromyricetin. Indeed, DFR genes have been shown to play an important role in determining the total anthocyanin content in A. thaliana26,27. Additionally, the expression level of the DFR gene TfDFR1 has been shown to be positively correlated with red pigment accumulation in the petals of tulip (Tulipa fosteriana)28. It has also been reported that the expression level of DFR positively correlates with the abundance of anthocyanins in peanut (Arachis hypogaea)29.
Flavonols provide another important co-pigment in the colorful organs of terrestrial plants, such as the yellow petals of Lathyrus chrysanthus30 and they also influence pollen tube growth31. Flavonols are derived from 2, 3-dihydroquercetin and their formation is catalyzed by FLS, which belongs to the 2-oxoglutarate dependent dioxygenase family4,32. Following the identification of an FLS gene from Petunia hybrida33, homologous FLS genes have been identified from A. thaliana, Solanum tuberosum, Matthiola incana, Malus domestica and Eustoma russellianum. In addition, it has been reported that increases in transcript levels of an FLS gene from satsuma mandarin (Citrus unshiu Marc.), CitFLS, in the fruit peel correlate with flavonol accumulation during fruit development34. Recently, it has been found that major floral color changes are a consequence of FLS expression in petunia (Petunia hybrida Vilm.), Lisianthus (Eustoma grandiflorum) and camellia (C. nitidissima)33,35,36,37. In contrast, an indirect effect of a camellia FLS gene (CnFLS1) on anthocyanin accumulation during floral coloration was suggested following an experiment where its overexpression in transgenic tobacco (N. benthamiana) plants resulted in an increase in flavonol content, but a reduction in anthocyanin levels in petals37.
Leaf color is a key determinant of the commercial value of many ornamental plant species; however, much remains to be learnt about the mechanisms of color formation in leaves at the molecular level. The study of pigmentation mechanisms in leaves is therefore significant for both breeding and genetic engineering of ornamental plants. An example of an important ornamental woody plant is Malus crabapple, which belongs to the Rosaceae, Malus Mill family. The numerous plant landscape species in this family provide an excellent source of research material for studying the mechanism of color formation and accumulation, due to their colorful leaves, flowers and fruits38. To date, little is known about the mechanism of anthocyanin and flavonol biosynthesis in ornamental crabapples.
In this current study, we investigated the function of the crabapple DFR (McDFR) and FLS (McFLS) genes in regulating leaf color in different cultivars. We overexpressed and silenced each gene to determine their interaction in controlling flavonol and anthocyanin biosynthesis and evaluated the gene expression ratio of McDFR and McFLS that is required for leaf color production. We also discuss the metabolic flux between McDFR and McFLS during flavonoid biosynthesis in leaves and fruit. We propose that the finding from this study will assist future attempts to enhance anthocyanin or flavonol accumulation in the leaves of target ornamental species by altering the balance between the McDFR and McFLS enzyme activities.
Results
The anthocyanin and flavonol content of the leaves of two crabapple cultivars
Two extreme leaf color cultivars, ‘Royalty’ and ‘Flame’, have ever-red and ever-green leaves, respectively. We evaluated the abundance of anthocyanins and flavonols in the leaves of these cultivars at 5 development stages of the crabapple leaf growing season by high-performance liquid chromatography (HPLC) (Fig. 2). The chromatography results showed that cyanidin 3-O-glucoside was the predominant anthocyanin and we found that that the major flavonols were quercetin derived compounds, such as quercetin 3-O-diglucoside, quercetin 3-O-glucoside and quercetin 3-O-glycosidase isomer (Fig. 2B). As shown in Fig. 2C, anthocyanin levels in the ever-red leaves of ‘Royalty’ were significantly higher than those in the ever-green leaves of ‘Flame’. A gradual decrease in anthocyanin content was observed in ‘Royalty’ leaves during their development, while anthocyanins were almost undetectable in ‘Flame’ leaves. In contrast, the abundance of flavonols increased during the development of ‘Flame’ leaves, except at stage 5.
Analysis of flavonoid accumulation in 5 developmental stages of leaves from the Malus crabapple cultivars ‘Royalty’ and ‘Flame’.
S1, 3 days after budding; S2, 9 days after budding; S3, 15 days after budding; S4, 21 days after budding; S5, 30 days after budding. (A) Five leaf developmental stages used for the analysis. (B) HPLC analysis of methanol extracts from crabapple leaf. A1, cyanidin 3-O-glucoside; F1, quercetin 3-O-diglucoside; F2, procyanidin dimer; F3, quercetin 3-O-glucoside; F4, quercetin 3-O-glycosidase isomer; F5, avicularin; F6, acetyl quercetin 3-O-glucoside; F7, acetyl quercetin 3-O-glycosidase isomer; F8, avicularin isomer; F9, quercetin 3-O-rhamnoside; F10, phloridzin. (C) The total anthocyanins content and total flavonols content in 5 developmental stages of leaves of the two crabapple cultivars. Error bars indicate the standard error of the mean ± SE of three replicate measurements. Different letters above the bars indicate significantly different values (P < 0.05) calculated using one-way analysis of variance (ANOVA) followed by a Duncan’s multiple range test.
The expression levels of anthocyanin and flavonol biosynthetic genes in the leaves of the two crabapple cultivars
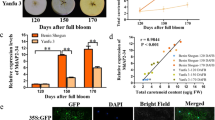
To gain insight into the relationship between the expression patterns of anthocyanin biosynthetic genes and anthocyanin/flavonol accumulation, the transcript levels of the key anthocyanin biosynthetic gene McDFR and the flavonol biosynthesis gene McFLS, were determined by qRT-PCR. The expression of McDFR decreased during the development of both ever-red and ever-green leaves (Fig. 3). In contrast, the transcript levels of McFLS showed an increase in the early developmental stages and then decreased in stages 4 and 5 in ever-red leaves (Fig. 2A), while only a slight decrease was seen in the last stage of the ever-green leaves (Fig. 3B). This suggested that McDFR expression positively correlated with anthocyanin accumulation during the development of ever-red leaves and that McFLS expression and flavonol accumulation were positively correlated in leaves of both cultivars.
The relative expression level of McDFR and McFLS transcripts at different development stages of ‘Royalty’ and ‘Flame’ leaves.
(A) ‘Royalty’. (B) ‘Flame’. Error bars indicate the standard error of the mean ± SE of three replicate measurements. Different letters above the bars indicate significantly different values (P < 0.05) calculated using one-way analysis of variance (ANOVA) followed by a Duncan’s multiple range test.
McDFR plays an important role in the anthocyanin biosynthetic pathway
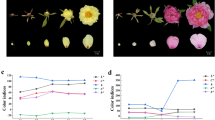
To further investigate the function of McDFR, we suppressed its expression in the leaves of the red-leafed crabapple cultivar ‘Royalty’ by virus-induced gene silencing (VIGS) using the TRV vector39. Leaves infected with the virus containing the TRV-McDFR construct began to exhibit a green color at 14 days post-infection (dpi), while a more severe phenotype was detected in the new buds of TRV-McDFR infected stem tips at 35 dpi (Fig. 4A). We next analyzed the variation in flavonoid content of the infected leaves by HPLC and observed that in the McDFR silenced areas of the leaves; the levels of anthocyanin were much lower than in non-silenced leaves, while the flavonol content showed a significant increase (Fig. 4B). We also confirmed, by qRT-PCR analysis, that the abundance of endogenous McDFR transcripts was greatly reduced in TRV-McDFR infected leaves. The expression levels of other flavonoid biosynthetic genes, namely CHS (McCHS) and F3′H (McF3′H) were similar in the control and infected leaves, while silencing of the McDFR gene increased the transcript levels of phenylalanine ammonia lyase (PAL; McPAL), CHI (McCHI), FSH (McF3H), FLS (McFLS), ANS (McANS) and UFGT (McUFGT) genes (Fig. 4C).
Transient expression of McDFR in crabapple.
McDFR expression was suppressed by VIGS using the vector pTRV2-McDFR in ‘Royalty’, or the gene was overexpressed using the vector pBI121-McDFR in ‘Strawberry Jelly’. Crabapple leaves injected with the empty TRV and pBI121 vectors and infiltration buffer were used as controls. (A) Phenotype of McDFR silenced or McDFR overexpressing ‘Royalty’ and ‘Strawberry Jelly’ leaves. (B) Anthocyanin and flavonol contents at the infiltration sites on crabapple leaves in μg/g fresh weight (FW). (C) Relative transcript expression levels in crabapple leaves around the infiltration sites were determined using qRT-PCR. Error bars indicate the standard error of the mean ± SE of three replicate measurements. Different letters above the bars indicate significantly different values (P < 0.05) calculated using one-way analysis of variance (ANOVA) followed by a Duncan’s multiple range test.
We also transiently over-expressed the McDFR gene in the stem tips of the ever-green cultivar ‘Strawberry Jelly’, which promoted anthocyanin accumulation at 20 dpi and a deep green coloration in most of the new buds (Fig. 4A). The anthocyanin content showed a slight increase in the McDFR-overexpressing plants (Fig. 4B) and we confirmed that these plants indeed had higher McDFR transcript levels in the new buds (Fig. 4C). We also detected an increase in the transcript levels of anthocyanin biosynthetic genes and a decrease in McFLS expression, compared with the non-transformed plants (Fig. 4C).
Collectively, these results indicated that McDFR expression is associated with red color formation in crabapple leaves and that changes in McDFR expression can affect the expression of downstream genes (e.g. ANS and UFGT) involved in the anthocyanin biosynthetic pathway.
McFLS is involved in flavonol biosynthesis in crabapple leaves
To confirm the prediction, based on sequence homology, that McFLS is a key flavonol biosynthetic gene, we suppressed its expression in the leaves of ‘Strawberry Jelly’ using the VIGS system and the TRV vector. Approximately 14 days after Agrobacterium infiltration, red coloration was seen in the margin and other areas of the infected leaves (Fig. 5A). HPLC analysis confirmed that the levels of anthocyanins were significantly higher in the silenced leaves than in control leaves infiltrated with TRV alone (Fig. 5B). Finally, as seen in Fig. 4C, the expression of McPAL, McDFR and McANS was up-regulated in infected leaves.
Transient expression of McFLS in crabapple.
McFLS expression was suppressed by VIGS using the vector pTRV2-McFLS in ‘Royalty’, or the gene was overexpressed using the vector pBI121-McFLS in ‘Strawberry Jelly’. Crabapple leaves injected with the empty TRV and pBI121 vectors and infiltration buffer were used as controls. (A) Phenotype of McFLS silenced or McFLS overexpressing ‘Strawberry Jelly’ and ‘Royalty’ leaves. (B) Anthocyanin and flavonol contents at infiltration sites of crabapple leaves in μg/g fresh weight (FW). (C) Relative transcript expression levels in crabapple leaves around the infiltration sites were determined using qRT-PCR. Error bars indicate the standard error of the mean ± SE of three replicate measurements. Different letters above the bars indicate significantly different values (P < 0.05) calculated using one-way analysis of variance (ANOVA) followed by a Duncan’s multiple range test.
We also transiently overexpressed McFLS in the stem tips of the ever-red leaves of ‘Royalty’. Crabapple stem tips transformed with 35S::McFLS developed new buds with a green color (Fig. 5A), indicating that anthocyanin synthesis was suppressed. This was confirmed by HPLC analysis, which also revealed an increase in flavonol content (Fig. 5B). As expected, we observed an increase in McFLS transcript levels in green buds infiltrated with 35S::McFLS compared to control leaves. This elevated expression of McFLS caused an approximately 1.6-fold decrease in McDFR transcript abundance and we observed lower levels of expression of other anthocyanin biosynthetic genes (Fig. 5C). To summarize, these results suggested that McFLS expression can promote flavonol accumulation by inhibiting the expression of McDFR and that McFLS has a competitive relationship with McDFR in the flavonol biosynthetic pathway.
Variation in the expression of McDFR and McFLS in apple fruit
To further characterize the roles of McDFR and McFLS, we infected apple (Malus × domestica ‘Fuji’) fruits with transgenic Agrobacterium harboring TRV-McDFR, TRV-McFLS, pBI121-McDFR or pBI121-McFLS constructs (Figs 6 and 7). A large increase in anthocyanin accumulation was observed at the sites of TRV-McFLS and pBI121-McDFR over-expression, while the areas of TRV-McDFR and pBI121-McFLS over-expression showed a yellow or white coloration (Figs 6A and 7A). HPLC quantification of the anthocyanin and flavonol content of the infected areas confirmed that the variation in flavonoid content correlated with the observed variation in fruit color (Figs 6B and 7B). Transcript expression analysis of the transiently expressing tissues further revealed that the up-regulation of McDFR expression, or the down-regulation of McFLS expression, was accompanied by a proportional increase in the expression levels of McDFR as well as some of the genes involved in anthocyanin biosynthesis (McCHS, McCHI, McF3H, McF3′H, McDFR, McANS and McUFGT) and a decrease in the expression levels of McFLS. Importantly, up-regulation of McFLS expression or down-regulation of McDFR expression resulted in fading leaf color and decrease McDFR gene expression (Figs 6C and 7C). Finally, we compared the relationship between the expression of McDFR and the content of anthocyanins and the results were consistent with the red color formation in leaves and fruit; as well as between the levels of McFLS and the content of flavonols (Figs 5 and 6).
Transient expression of McDFR in apple fruit.
McDFR expression was suppressed in apple fruit using the vector pTRV2-McDFR, or the gene was overexpressed using the vector pBI121-McDFR. Apple fruit injected with the empty TRV and pBI121 vectors and infiltration buffer were used as controls. (A) Phenotype of McDFR silenced or overexpressing McDFR apple peels. (B) Anthocyanin and flavonol contents at the infiltration sites of apple peels in μg/g fresh weight (FW). (C) Relative expression levels around the infiltration sites were determined using qRT-PCR. Error bars indicate the standard error of the mean ± SE of three replicate measurements. Different letters above the bars indicate significantly different values (P < 0.05) calculated using one-way analysis of variance (ANOVA) followed by a Duncan’s multiple range test.
Transient expression of McFLS in apple fruit.
McFLS expression was suppressed in apple fruit using the vector pTRV2- McFLS, or overexpressed using the vector pBI121- McFLS. Apple fruit injected with the empty TRV and pBI121 vectors and infiltration buffer were used as controls. (A) Phenotype of McFLS silenced or McFLS overexpressing apple peels. (B) Anthocyanin and flavonol contents at the infiltration sites of apple peels in μg/g fresh weight (FW). (C) Relative expression levels were determined using qRT-PCR around the infiltration sites. Error bars indicate the standard error of the mean ± SE of three replicate measurements. Different letters above the bars indicate significantly different values (P < 0.05) calculated using one-way analysis of variance (ANOVA) followed by a Duncan’s multiple range test.
Discussion
Due to the central role that the DFR enzyme plays in the anthocyanin biosynthetic pathway, DFR genes have been studied in several monocotyledonous and dicotyledonous species, such as Forsythia intermedia40, Torenia fournieri31, Triticum aestivum41, Vitis vinifera42 and Ascocenda spp.43 and in some cases at the transcriptional level44. The key structural gene, FLS, completes the last step of flavonol synthesis. To our knowledge, although FLS and DFR have been identified and characterized in many land plant species, these genes have yet to be studied in ornamental crabapples. There is growing evidence that anthocyanins and flavonols to contribute to the ornamental and economic value of crabapples, which have highly colorful leaves, fruits and flowers38. In this study we explored the expression and function of McDFR and McFLS in the biosynthesis and accumulation of anthocyanins and flavonols.
McDFR and McFLS expression are coordinated and correlate with the accumulation of anthocyanins and flavonols
In this study, the content of anthocyanins in the leaves of two crabapple cultivars was evaluated and we observed that it increased in parallel with red pigmentation in several organs/tissues. Furthermore, the anthocyanin levels showed a positive correlation with the expression of McDFR, but a negatively correlation with the expression of McFLS (Table 1). This result is congruent with a recent study showing a negative correlation of CnFLS1 expression and anthocyanin synthesis during floral coloration in the petals of transgenic tobacco expressing this gene37. We infer from these observations that there is a competitive relationship between McDFR and McFLS in flavonoid biosynthesis.
The function of McDFR and McFLS in flavonoid biosynthesis
Genetic transformation has been used to test the functions of several genes in the flavonoid biosynthetic pathway in model experimental plants, such as tobacco and A. thaliana, as well as in some crop species. For example, overexpression of petunia CHI in tomato fruit was reported to lead to an ~65% increase in flavonol levels45, while silencing of a CHS gene in apple fruit resulted in changes in growth and developmental phenotypes46. In Gentiana triflora and apples (Malus spp.), the silencing of an ANS gene caused a reduction in anthocyanin content and, consequently, a much weaker color11. However, few studies to date have focused on genetic transformation using DFR or FLS genes and none has targeted woody ornamental species, such as crabapple.
Virus-induced gene silencing (VIGS) is a technology that allows the analysis of genes function by suppressing the expression of target genes. In a previous study, we assessed the effect of gene silencing in several plant species, including Nicotiana benthamiana, rose (Rose hybrida) and strawberry (Fragaria × ananassa)47,48,49. Here, we used tissue cultured seedling buds from different crabapple cultivars (‘Royalty’, ‘Strawberry Jelly’) to assess the function of McDFR and McFLS through VIGS and overexpression approaches. We determined that silencing of McFLS or overexpression of McDFR promoted the accumulation of anthocyanins, while the opposite results were obtained when McFLS was overexpressed or McDFR expression was silenced. In addition, the abundance of flavonols increased when McFLS was overexpressed or McDFR was silenced. However, it is interesting that levels of flavonols were slightly elevated in transgenic crabapple leaves that were transiently overexpressing McDFR or in which McFLS expression was silenced. This phenomenon may be explained if McFLS was still expressed but at lower levels degree in McDFR overexpressed or McFLS silenced crabapple plants. In this scenario the lower expression level of McFLS in these transgenic crabapple plants may have resulted in reduced accumulation flavonol, but not a complete block in flavonol biosynthesis. Moreover, correlation coefficients indicated that the levels of expression of McDFR negatively correlated with those of McFLS, as well as with the abundance of anthocyanins and flavonols (Table 1). We propose that there is a competitive relationship between the expression McDFR and McFLS that results in the production of different classes of flavonoid compounds (i.e. anthocyanins or flavonols) (Figs 4, 5, 6, 7).
Since dihydroflavonols are substrates for DFR and FLS, they lie at an important branch point in flavonoid biosynthesis, where precursor substrates are channeled toward either anthocyanin or flavonol production. In this regard, the regulation of DFR expression and the competing DFR and FLS activities may be particularly important. We conclude that the expression of McDFR and McFLS may represent a key mechanism for regulating color in crabapple leaves.
A possible explanation for the expression patterns of McDFR and McFLS in two contrasting varieties
R2R3-MYB transcription factors (TFs), which belong to one of the largest plant TF families, are known to be involved in regulating the biosynthesis of anthocyanins and flavonols in A. thaliana50. A previously study reported that over-expression of the A. thaliana TF AtMYB12 in tobacco (Nicotiana tabacum) resulted in higher levels of flavonols and increased expression of the FLS gene51. Moreover, when AtMYB12 was over-expressed in a tissue-specific manner in tomato, the flavonol biosynthesis pathway was activated51. However, expression of the DFR gene was not induced in AtMYB12 and AtMYB111 over-expressing transgenic plants, as well as in AtMYB111 transgenic lines1,44,51. In apple and crabapple, MYB10, which regulates anthocyanin accumulation and coloration of various organ (e.g. fruit, petals and leaves), can activate the expression of DFR and bind to several the promoters of several anthocyanin biosynthetic genes52,53. In grape berries, expression of the regulatory gene VvMYBF1 is light inducible and is involved in the control of VvFLS1 transcription and flavonol synthesis in fruit54. Thus, we speculated that MYB TFs may similarly regulate flavonoid biosynthesis in crabapple and that their expression levels may vary at different development stages or in response to different environment conditions. Moreover, changes in the transcript levels of MYB TFs control the biosynthesis of flavonoids, by regulating the expression of various members of the flavonoid biosynthetic pathway. We propose that MYB TFs promote anthocyanin biosynthesis by increasing the transcript levels of DFR during fruit or leaf development, leading to red coloration. However, in response to environmental stresses, MYB TFs activate the transcription of FLS resulting in increased production of flavonols.
Conclusion
In this study, we investigated the functions of McDFR and McFLS in regulating leaf color in different crabapple cultivars. We demonstrated that the competitive relationship between the expression of McDFR and McFLS is important for anthocyanin and flavonol synthesis. In addition, the expression of McDFR and McFLS correlated with anthocyanin and flavonol accumulation, as well as with leaf color. The work described in this report may suggest strategies to genetically modify ornamental plants in order to enhance or modulate flavonoid accumulation.
Methods
Plant material and growth conditions
The plant material used included three Malus crabapple cultivars: (1) Malus cv. ‘Royalty’, an ever-red leaf cultivar; (2) Malus cv. ‘Flame’, an ever-green leaf cultivar. Five year old trees of these cultivars grafted on M. hupehensis were planted in the Crabapple Germplasm Resources Nursery in Beijing University of Agriculture; and (3) Malus cv. ‘Strawberry Jelly’, an ever-green leaf cultivar, the explants of which were harvested from one-year old branches before spring bud germination, cultured on Murashige and Skoog medium supplemented with 0.1 mg/L 6-Benzylaminopurine (6-BA) and 2 mg/L (2,4-dichlorophenoxy) acetic acid (2,4-D) at 23 °C with a 16 h light (200 μmol s–1 m–2) /8 h dark period. Plants were grown in a greenhouse at 27 °C under constant illumination.
Leaves of ‘Royalty’ and ‘Flame’ were collected at five different developmental stages (Fig. 1A,B) for gene expression analyses and anthocyanin and flavonol quantification. Wild-type ‘Royalty’ and ‘Strawberry’ seedlings were grown in a greenhouse, as above. Apple (Malus × domestica ‘Fuji’) fruits were used for analysis of McDFR and McFLS expression. All samples were frozen in liquid nitrogen upon collection and stored at −80 °C until further use.
Construction of VIGS vectors and Agrobacterium-mediated infiltration of crabapple
The coding sequences of McDFR (GenBank: FJ817487) and McFLS (GenBank: KF495602) have previously been deposited in the NCBI (National Center for Biotechnology Information) database. Fragments for the pTRV2-McDFR (540 bp) and pTRV2-McFLS (490 bp) constructs were amplified by PCR, using gene-specific primers, from a cDNA library derived from Malus crabapple leaves (cv. ‘Royalty’) using Taq DNA polymerase (TAKARA BIOTECH) according to the manufacturer’s instructions. The PCR primers used are shown in Table S1. Virus-induced gene silencing vectors carrying the target gene fragments, as well as pTRV1 and pTRV247,48,49, were transformed into Agrobacterium tumefaciens strain GV3101 competent cells using a freeze-thaw method55 and selected on kanamycin- rifampicin-containing (50 mg/L) LB (Luria Bertani media) plates. Positive clones were verified by restriction enzyme digestion and by sequencing the vector-insert junctions. The harvested bacterial cells were then resuspended to an OD600 of 0.5 in infiltration medium (10 mM 2-morpholinoethanesulfonic acid [MES], 200 mM acetosyringone and 10 mM MgCl2) and incubated at room temperature for 3 h. Before infiltration, bacteria carrying pTRV1 and pTRV2 were mixed in a 1: 1 volume ratio.
For vacuum infiltration, whole plants were submerged in the Agrobacterium suspension and subjected to a vacuum (−25 kPa). When the rate of air bubbles being released from the plants started to decrease, the vacuum was released quickly to allow bacteria to enter the plant tissues. The vacuum treatment time varied from 30 s up to 3 min, depending on the vacuum source used. After vacuum infiltration, plants were rinsed with sterile water and cultured on Murashige and Skoog medium. Fifteen plants from each cultivar were treated and ‘Royalty’ was used for silencing of McDFR expression, while ‘Strawberry Jelly’ was used for silencing of McFLS expression. All experiments were repeated three times.
Overexpression of McDFR and McFLS in crabapple leaves and fruits
The full length McDFR and McFLS open reading frames (ORFs) were cloned from the cDNA library described above and inserted into the pBI121 vector53 using the XbaI and SacI sites. Primers used for these constructs are shown in Table S1. Transient expression in Malus crabapple leaves was performed using the ‘Strawberry’ Jelly cultivar and Agrobacterium-mediated transformation, as described above. Agrobacterium cells containing the different constructs were harvested and resuspended in infiltration buffer (10 mM MES, 0.2 mM acetosyringone and 10 mM MgCl2) to a final concentration of OD600 = 0.5. Vacuum infiltration was performed as described above and infiltration with an empty vector was used as a negative control. Seven days after infiltration, the infected leaves and fruit were collected to observe phenotypic features and to evaluate differences in expression.
RNA extraction
To analyze the effects of VIGS and overexpression on target genes expression, tissue samples from areas showing the silencing and enhancing phenotypes were collected. For controls, corresponding samples were collected from tissues infected by Agrobacterium carrying vectors with no host gene fragment insert, or from non-infected plants. Samples from three independent biological replicates were analyzed. Total RNA was extracted from crabapple leaves using the RNA plant plus Reagent (TIANGEN BIOTECH) according to the manufacturer’s instructions. DNase (TIANGEN BIOTECH) treatment was performed to remove any genomic DNA according to the manufacturer’s instructions. First-strand cDNA was synthesized from total RNA using the Reverse Transcriptase M-MLV (RNase H−) kit (TaKaRa).
Quantitative RT-PCR analysis
qRT-PCR was performed using the SYBR® Premix Ex TaqTM II (Perfect Real Time) (TaKaRa, Ohtsu, Japan) and the CFX96TM Real Time System (Bio-Rad, USA). The PCR amplification conditions were as previously described56 and transcript levels were determined by relative quantification using the Malus 18S ribosomal RNA gene (DQ341382) as the internal control and the 2^ (−∆∆CT) analysis method was applied. Specific primers (Table S1) for semi-quantitative RT-PCR and qRT-PCR analysis were designed using the primer 5 software57.
HPLC analysis
Crabapple leaf samples (approximately 0.8–1.0 g fresh weight) were subjected to extraction with 10 mL extraction solution (methanol: water: formic acid: trifluoroacetic acid= 70: 27: 2: 1)58 at 4 °C in the dark for 72 h, shaking every 6 h. The supernatant was isolated by filtration through filter paper and a further filtration through a 0.22 μm MilliporeTM filter (Billerica, MA, USA). For the HPLC analysis, trifluoroacetic acid: formic acid: water (0.1: 2: 97.9) was used as mobile phase A and trifluoroacetic acid: formic acid: acetonitrile: water (0.1: 2: 48: 49.9) was used as mobile phase B. The gradients used were as follows: 0 min, 30% B; 10 min, 40% B; 50 min, 55% B; 70 min, 60% B; 30 min, 80% B. Detection was performed at 520 nm for anthocyanin and 350 nm for flavonol58, respectively. All samples were analyzed in three biological triplicates (extracted from three different batches of leaves).
In this study, we employed HPLC-ESI (±)-MS2 analysis to identify the kinds of compounds by standards and comparing their spectroscopic data to literature59,60. Cyanidin-3-O-glucoside, quercetin-3-O-glucoside, avicularin, phloridzin, quercetin (Sigma-Aldrich, Germany), Procyanidin B2 (Sigma-Aldrich, UK) was used as standards.
Additional Information
How to cite this article: Tian, J. et al. The Balance of Expression of Dihydroflavonol 4-reductase and Flavonol Synthase Regulates Flavonoid Biosynthesis and Red Foliage Coloration in Crabapples. Sci. Rep. 5, 12228; doi: 10.1038/srep12228 (2015).
References
Misra, P. et al. Modulation of transcriptome and metabolome of tobacco by Arabidopsis transcription factor, AtMYB12, leads to insect resistance. Plant Physiol. 152, 2258–2268 (2010).
Page, M., Sultana, N., Paszkiewicz, K., Florance, H. & Smirnoff, N. The influence of ascorbate on anthocyanin accumulation during high light acclimation in Arabidopsis thaliana: further evidence for redox control of anthocyanin synthesis. Plant Cell Environ. 35, 388–404 (2012).
Wojdyło, A., Oszmianski, J. & Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 105, 940–949 (2007).
Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology and biotechnology. Plant Physiol. 126, 485–493 (2001).
Martens, S., Preuss, A. & Matern, U. Multifunctional flavonoid dioxygenases: flavonol and anthocyanin biosynthesis in Arabidopsis thaliana L. Phytochemistry 71, 1040–1049 (2010).
Kobayashi, H., Oikawa, Y., Koiwa, H. & Yamamura, S. Flower-specific gene expression directed by the promoter of a chalcone synthase gene from Gentiana triflora in Petunia hybrida. Plant Sci. 131, 173–180 (1998).
Tanaka, Y. et al. Molecular and biochemical characterization of three anthocyanin synthetic enzymes from Gentiana triflora. Plant Cell Physiol. 37, 711–716 (2006).
Fujiwara, H. et al. cDNA cloning, gene expression and subcellular localization of anthocyanin 5-aromatic acyltransferase from Gentiana triflora. Plant J. 16, 421–431 (2001).
Jaakola, L. et al. Expression of genes involved in anthocyanin biosynthesis in relation to anthocyanin, proanthocyanidin and flavonol levels during bilberry fruit development. Plant Physiol. 130, 729–739 (2002).
Park, J. S. et al. EST analysis of genes involved in secondary metabolism in Camellia sinensis (tea), using suppression subtractive hybridization. Plant Sci. 166, 953–961 (2004).
Nakatsuka, T., Nishihara, M., Mishiba, K. & Yamamura, S. Temporal expression of flavonoid biosynthesis-related genes regulates flower pigmentation in gentian plants. Plant Sci. 168, 1309–1318 (2005).
Fukuchi-Mizutani, M. et al. Biochemical and molecular characterization of a novel UDP-glucose: anthocyanin 3-O-glucosyltransferase, a key enzyme for blue anthocyanin biosynthesis, from gentian. Plant Physiol. 132, 1652–1663 (2003).
Moriguchi, T., Kita, M., Tomono, Y., Endo-Inagaki, T. & Omura, M. Gene expression in flavonoid biosynthesis: correlation with flavonoid accumulation in developing citrus fruit. Plant Physiol. 111, 66–74 (2008).
Han, Y. P., Vimolmangkang, S., Soria-Guerra, R. E. & Korban, S. S. Introduction of apple ANR genes into tobacco inhibits expression of both CHI and DFR genes in flowers, leading to loss of anthocyanin. J. Exp. Bot. 63, 2437–2447 (2012).
Dare, A. P. et al. Phenotypic changes associated with RNA interference silencing of chalcone synthase in apple (Malus × domestica). Plant J. 74, 398–410 (2013).
Holton, T. A. & Cornish, E. C. Genetics and biochemistry of anthocyanin biosynthesis. Plant Cell. 7, 1071–1083 (1995).
Grotewold, E., Athma, P. & Peterson, T. Alternatively spliced products of the maize P gene encode proteins with homology to the DNA-binding domain of myb-like transcription factors. Proc. Natl. Acad. Sci. 88, 4587–4591 (1991).
Lepiniec, L. et al. Genetics and biochemistry of seed flavonoids. Plant Biol. 57, 405–430 (2006).
Aharoni, A. et al. The strawberry FaMYB1 transcription factor suppresses anthocyanin and flavonol accumulation in transgenic tobacco. Plant J. 28, 319–332 (2001).
Gou, J. Y., Felippes, F. F., Liu, C. J., Weigel, D. & Wang, J. W. Negative regulation of anthocyanin biosynthesis in Arabidopsis by a miR156-targeted SPL transcription factor. Plant Cell. 23, 1512–1522 (2011).
Stracke, R. et al. Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling. Plant J. 50, 660–677 (2007).
Matsui, K., Umemura, Y. & Ohme-Takagi, M. AtMYBL2, a protein with a single MYB domain, acts as a negative regulator of anthocyanin biosynthesis in Arabidopsis. Plant J. 55, 954–967 (2008).
Zhu, Y., Evans, K. M. & Peace, C. Utility testing of an apple skin color MdMYB1 marker in two progenies. Mol. Breeding 27, 525–532 (2011).
Espley, R. V. et al. Red colouration in apple fruit is due to the activity of the MYB transcription factor, MdMYB10. Plant J. 49, 414–427 (2007).
Ban, Y. et al. Isolation and functional analysis of a MYB transcription factor gene that is a key regulator for the development of red coloration in apple skin. Plant Cell Physiol. 48, 958–970 (2007).
Shirley, B. W., Hanley, S. & Goodman, H. M. Effects of ionizing radiation on a plant genome: Analysis of two Arabidopsis transparent testa mutations. Plant Cell. 4, 333–347 (1992).
Shirley, B. W. et al. Analysis of Arabidopsis mutants deficient in flavonoid biosynthesis. Plant J. 8, 659–671 (1995).
Yuan, Y., Ma, X. H., Shi, Y. & Tang, D. Q. Isolation and expression analysis of six putative structural genes involved in anthocyanin biosynthesis in Tulipa fosteriana. Sci. Hor. 153, 93–102 (2013).
Guo, F. D., Xia, H. Y. M. & Wang, X. J. Cloning of dihydroflavonol 4-reductase gene (DFR) from peanut (Arachis hypogaea L.) and its expression analysis. J Agri. Biotechnol. 19, 816–822 (2011).
Markham, K. R. & Hammett, K. R. W. The basis of yellow coloration in Lathyrus-Aphaca flowers. Phytochemistry 37, 163–165 (1994).
Aida, R., Yoshida, K., Kondo, T., Kishimoto, S. & Shibata, M. Copigmentation gives bluer flowers on transgenic torenia plants with the antisense dihydroflavonol 4-reductase gene. Plant Sci. 160, 49–56 (2000).
Winkel, B. S. J. Metabolic channeling in plants. Annu. Review Plant Biol. 55, 85–107 (2004).
Holton, T. A., Brugliera, F. & Tanaka, Y. Cloning and expression of flavonol synthase from Petunia hybrida. Plant J. 4, 1003–1010 (1993).
Moriguchia, T. et al. Flavonol synthase gene expression during citrus fruit development. Physilo. Plantarum. 114, 251–258 (2002).
Tsuda, S. et al. Molecular breeding of flower color of Torenia hybrida. Curr. Plant Sci. Biot. 36, 613–616 (1999).
Nielsen, K. et al. Antisense flavonol synthase alters copigmentation and flower color in lisianthus. Mol. Breed. 9, 217–229 (2002).
Zhou, X. W. et al. Functional analyses of a flavonol synthase-like gene from Camellia nitidissima reveal its roles in flavonoid metabolism during floral pigmentation. J. Bio. Sci. 38, 593–604 (2013).
Tian, J., Shen, H. X., Zhang, J., Song, T. T. & Yao, Y. C. Characteristics of chalcone synthase promoters from different leaf-color Malus crabapple cultivars. Sci. Hort. 129, 449–458 (2011).
Liu, Y., Schiff, M. & Dinesh-Kumar, S. P. Virus-induced gene silencing in tomato. Plant J. 31, 777–786 (2002).
Rosati, C., Cadic, A., Duron, M., Renou, J. P. & Simoneau, P. Molecular cloning and expression analysis of dihydroflavonol 4-reductase gene in flower organs of Forsythia x intermedia. Plant Mol. Biol. 35, 303–311 (1997).
Himi, E. & Noda, K. Isolation and location of three homologous dihydroflavonol 4-reductase (DFR) genes of wheat and their tissue-dependent expression. J. Exp. Bot. 55, 365–375 (2004).
Zhang, P. et al. Molecular cloning of dihydroflavonol 4-reductase gene from grape berry and preparation of an anti-DFR polyclonal antibody. Vitis. 47, 141–145 (2008).
Kunu, W., Thanonkeo, S. & Thanonkeo, P. Cloning and expression analysis of dihydroxyflavonol 4-reductase (DFR) in Ascocenda spp. Afr. J. Biotechnol. 11, 12702–12709 (2012).
Pandey, A., Misra, P., Bhambhani, S., Bhatia, C. & Trivedi, P. K. Expression of Arabidopsis MYB transcription factor, AtMYB111, in tobacco requires light to modulate flavonol content. Sci. Rep. 4, 5018; 10.1038/srep05018 (2014).
Muir, S. R. et al. Overexpression of petunia chalcone isomerase in tomato results in fruit containing increased levels of flavonols. Nature Biotechnol. 19, 470–474 (2001).
Dare, A. P. et al. Phenotypic changes associated with RNA interference silencing of chalcone synthase in apple (Malus domestica). Plant J. 74, 398–410 (2013).
Tian, J. et al. TRV-GFP: a modified tobacco rattle virus vector for efficient and visualizable analysis of gene function. J. Exp. Bot. 65, 311–322 (2013).
Tian, J., Cheng, L., Han, Z. Y. & Yao, Y. C. Tobacco rattle virus mediated gene silencing in strawberry plants. Plant Cell Tiss. Organ Cult. 120, 1131–1138 (2015).
Tian, J. et al. McMYB10 regulates coloration via activating McF3′H and later structural genes in ever-red leaf crabapple. Plant Biotechnol. J. 10.1111/pbi.12331 (2015).
Riechmann, J. L. et al. Arabidopsis transcription factors: genome-wide co mparative analysis among eukaryotes. Science 290, 2105–2110 (2000).
Luo, J. et al. AtMYB12 regulates caffeoyl quinic acid and flavonol synthesis in tomato: expression in fruit results in very high levels of both types of polyphenol. Plant J. 56, 316–326 (2008).
Ravaglia, D. et al. Transcriptional regulation of flavonoid biosynthesis in nectarine (Prunus persica) by a set of R2R3 MYB transcription factors. BMC Plant Biol. 13, 68 (2013).
Jiang, R., Tian, J., Song, T. T., Zhang, J. & Yao, Y. C. The Malus crabapple transcription factor McMYB10 regulates anthocyanin biosynthesis during petal coloration. Sci. Hortic. 166, 42–49 (2014).
Czemmel, S. et al. The grapevine R2R3-MYB transcription factor VvMYBF1 regulates flavonol synthesis in developing grape berries. Plant Physiol. 151, 1513–1530 (2009).
Yan, H. X. et al. Sprout vacuum-infiltration: a simple and efficient agroinoculation method for virus-induced gene silencingin diverse solanaceous species. Plant Cell Rep. 31, 1713–1722 (2012).
Shen, H. X. et al. Isolation and expression of McF3H gene in the leaves of crabapple. Acta. Physiol. Plant 34, 1353–1361 (2012).
Tai, D. Q., Tian, J., Zhang, J., Song, T. T. & Yao, Y. C. A Malus crabapple chalcone synthase gene, McCHS, regulates red petal color and flavonoid biosynthesis. Plos One 10: e110570 (2014).
Revilla, E. & Ryan, J. M. Analysis of several phenolic compounds with potential antioxidant properties in grape extracts and wines by high-performance liquid chromatography–photodiode array detection without sample preparation. J. Chromatogr. A. 881, 461–469 (2000).
Piero, A. R. L., Puglist, I. & Petrone, G. Gene characterization, analysis of expression and in vitro synthesis of dihydroflavonol 4-reductase from Citrus sinensis (L.). Osbeck phytochem. 67, 684–695 (2006).
Yildiz, M. et al. Expression and mapping of anthocyanin biosynthesis genes in carrot. Theor. Appl. Genet. 126, 1689–1702 (2013).
Acknowledgements
We thank the Fruit Tree Key Laboratory at the Beijing University of Agriculture. We also thank the Beijing Key Laboratory for Agricultural Application and New Technique for providing experimental resources. We are grateful to all the technicians in the BUA Crabapple Germplasm Resource Garden. We thank PlantScribe (www.plantscribe.com) for carefully editing this manuscript. Financial support was provided by the National “Twelfth Five-Year” Plan for Science & Technology Support (Project No. 2013BAD02B01-4), National Natural Science Foundation of China (31301762), the Project of Construction of Innovative Teams and Teacher Career Development for Universities and Colleges Under Beijing Municipality (IDHT20140509), Beijing Municipal Commission of Education Science and Technology Promotion Plan (PXM2014-014207-000081) and Scientific Research Improvement Project of Beijing University of Agriculture (GZL2015003). The funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: J.T. and Y.Y. Performed the experiments: Z.H. and Y.H. Analyzed the data: J.Z. and T.S. Contributed reagents/materials/analysis tools: Y.Y. and T.S. Wrote the paper: J.T., Y.Y. and Z.H.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Tian, J., Han, Zy., Zhang, J. et al. The Balance of Expression of Dihydroflavonol 4-reductase and Flavonol Synthase Regulates Flavonoid Biosynthesis and Red Foliage Coloration in Crabapples. Sci Rep 5, 12228 (2015). https://doi.org/10.1038/srep12228
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep12228
This article is cited by
-
Transcriptome profiling of cashew apples (Anacardium occidentale) genotypes reveals specific genes linked to firmness and color during pseudofruit development
Plant Molecular Biology (2022)
-
Integrated metabolome and transcriptome analysis of the anthocyanin biosynthetic pathway in relation to color mutation in miniature roses
BMC Plant Biology (2021)
-
Comparative transcriptome analysis reveals key genes associated with pigmentation in radish (Raphanus sativus L.) skin and flesh
Scientific Reports (2021)
-
Genome-Wide Investigation of Major Enzyme-Encoding Genes in the Flavonoid Metabolic Pathway in Tartary Buckwheat (Fagopyrum tataricum)
Journal of Molecular Evolution (2021)
-
Metabolome and transcriptome profiling of Theobroma cacao provides insights into the molecular basis of pod color variation
Journal of Plant Research (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.