Abstract
Understanding and engineering the flow-response of complex and non-Newtonian fluids at a molecular level is a key challenge for their practical utilisation. Here we demonstrate the coupling of microfluidics with small angle neutron scattering (SANS). Microdevices with high neutron transmission (up to 98%), low scattering background ( ), broad solvent compatibility and high pressure tolerance (≈3–15 bar) are rapidly prototyped via frontal photo polymerisation. Scattering from single microchannels of widths down to 60 μm, with beam footprint of 500 μm diameter, was successfully obtained in the scattering vector range 0.01–0.3 Å−1, corresponding to real space dimensions of
), broad solvent compatibility and high pressure tolerance (≈3–15 bar) are rapidly prototyped via frontal photo polymerisation. Scattering from single microchannels of widths down to 60 μm, with beam footprint of 500 μm diameter, was successfully obtained in the scattering vector range 0.01–0.3 Å−1, corresponding to real space dimensions of  . We demonstrate our approach by investigating the molecular re-orientation and alignment underpinning the flow response of two model complex fluids, namely cetyl trimethylammonium chloride/pentanol/D2O and sodium lauryl sulfate/octanol/brine lamellar systems. Finally, we assess the applicability and outlook of microfluidic-SANS for high-throughput and flow processing studies, with emphasis of soft matter.
. We demonstrate our approach by investigating the molecular re-orientation and alignment underpinning the flow response of two model complex fluids, namely cetyl trimethylammonium chloride/pentanol/D2O and sodium lauryl sulfate/octanol/brine lamellar systems. Finally, we assess the applicability and outlook of microfluidic-SANS for high-throughput and flow processing studies, with emphasis of soft matter.
Similar content being viewed by others
Introduction
The advent of microfluidics1 and rapid prototyping2,3 provides opportunities to design and generate well-defined flow fields with unprecedented flexibility, ranging from extension to rotation and including pure shear4. Despite the general absence of non-linearity associated with inertial forces, fluids confined within microchannels (1–1000 μm), exhibit unexpected and fascinating behaviour5. While microflows are generally characterised by low Reynolds numbers, resulting in diffusive and thus slow mixing, simple designs of both continuous and multiphase flows can reduce mixing times to the sub-millisecond range6,7,8 and have enabled major advances in, for example, the study of fast reaction kinetics6 or the rapid screening of protein crystallisation9. Further, microfluidic approaches are generally high throughput, either sequential or parallel and require minimal sample consumption, in the pL-μL range6,10. Complex or non-Newtonian fluids, in particular, can exhibit dramatic microflow response due to the commensurability of characteristic time and length scales5. Microfluidic methods can not only precisely quantify their rheological behaviour11 but even access hitherto unexplored phenomena that emerge, for example, when large Deborah (De) numbers and low Reynolds (Re) numbers are achieved simultaneously12. Soft matter generally responds strongly to flow perturbations exhibiting shear thinning or thickening, thixotropy or rheopexy, but also flow-induced transitions, such as gelation or phase separation13,14,15,16,17. The understanding of these non-equilibrium phenomena and mapping of ‘flow phase diagrams’17,18 can therefore be greatly expanded with the plethora of flow types and magnitudes available via rapid prototyping microfluidics.
Small angle scattering is a unique, non-invasive, molecular and nanoscale probe of such structural and kinetic transitions19,20 and its coupling with microfluidics is thus highly desirable. Complementing an array of in situ microscopic, spectroscopic and rheological approaches5,21,22, microfluidic-SAXS23,24,25,26,27,28 has been demonstrated in the high throughput mapping of protein conformation29, resolution of sub-ms kinetics of protein folding23, nucleation and growth of nanoparticles30, or the study of complex fluids under flow, specifically lamellar or particulate solutions under shear and extensional flows25,31,32. While synchrotron SAXS sources offer high brilliance (~1012,13,14 photons s−1 at the sample) and small beam sizes (100 μm to sub-μm), scattering contrast in multicomponent soft and biological systems, generally comprising low atomic number species, is fixed and somewhat limited. Small Angle Neutron Scattering (SANS)19,33 contrast, on the other hand, arises from nuclear interactions which vary markedly for light elements and between isotopes and significantly between hydrogen and deuterium. Selective deuteration has enabled major advances in soft matter science, from the elucidation of polymer conformation in melts34, the reptation mechanism35 or exchange kinetics in micelles36 to name a few33,37. Neutrons also offer large material penetration (approximately 90% for 1 cm of aluminium). However, SANS suffers from considerably lower fluxes (~108 neutrons cm−2 s−1 ) compared to SAXS and limited focussing and collimation, generally requiring longer acquisition times and larger beam sizes, typically 1–1000 s and 10 mm in diameter, respectively. While rheo-SANS is now a well-established technique38, flow is generally limited to Couette or cone/plate-plate geometries, stopped flow for rapid mixing and kinetics39, or custom made flow cells including Poiseuille40, cross-flow41, contraction slit42 and screw-extruder geometries43, with characteristic dimensions in the mm to cm range, which can now be significantly relaxed in microfluidics44. However, despite its great potential, the coupling of microfluidics and SANS appears challenging, due to the small scattering volumes associated with microchannel dimensions (≈100 μm) and unfavourable signal-background ratio by common microfluidic matrices (e.g., polydimethyl siloxane elastomers or other hydrogenated polymers, often thermoplastics). In this letter, we tackle this challenge, benefitting from the sustained improvement of neutron sources and advances in rapid-prototyping of microdevices45,46,47,48 and investigate the molecular reorganisation of model concentrated surfactant mixtures under flow.
Results
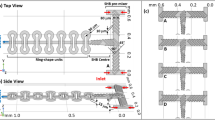
Microdevices were fabricated by single-step frontal photo polymerisation (FPP) of a thiol-ene copolymer45,48 within thin borosilicate or quartz plates, with a mechanically reinforced frame to impart rigidity and enable port connection and operation. Lateral microchannel dimensions from 2 mm to as small as 60 μm are readily achieved, with depths of 100–1000 μm and a range of flow geometries including periodic contraction-expansion flows, cross-slots and channel height gradients are fabricated. Single channel SANS measurements were demonstrated at the D22 spectrometer at ILL, Grenoble, configured to yield a scattering vector q-range of 0.01–0.33 Å−1 and typical flux of 5 104 neutron/s at 6 Å incident wavelength (Supplementary Table 1), with typical beam diameter of 500 μm (thus over illuminating the channels). Two concentrated surfactant mixtures were chosen for this study, forming lyotropic lamellar phases with an additional hydrophobic (oil) component, due to their ubiquitous practical application and flow response49. Lamellar phases derive their microstructure from a competition between interfacial curvature energy and entropy and are highly responsive to flow alignment and, in specific flow conditions, hydrodynamic instabilities result in the formation of long-lived metastable phases (e.g. onion phase17). We select well-known systems of cetyl trimethylammonium chloride(CTAC)/pentanol/D2O25 and sodium lauryl sulfate (SDS)/octanol/d-brine17,18 with characteristic spacings of  and 150 Å (detailed in Supplementary Information). The experimental setup is illustrated in figure 1(a) and an optical micrograph of a periodic 6-constriction microdevice is shown in (c). Contraction-expansion and crossslot geometries yield primarily extensional flows, which are ubiquitous in the processing of non-Newtonian fluids, ranging from injection moulding, to spraying or spray drying, or continuous flow processing of formulations (including the aforementioned lamellar phases). The SANS experiment starts by generating a spatial microchannel map by fine xy neutron transmission scans, illustrated in figure 1(b, d, e), followed by the spatiotemporal mapping at selected positions. Two-dimensional SANS acquisitions as short as 1 s are able to resolve the spatial flow response of these complex fluids at the molecular level.
and 150 Å (detailed in Supplementary Information). The experimental setup is illustrated in figure 1(a) and an optical micrograph of a periodic 6-constriction microdevice is shown in (c). Contraction-expansion and crossslot geometries yield primarily extensional flows, which are ubiquitous in the processing of non-Newtonian fluids, ranging from injection moulding, to spraying or spray drying, or continuous flow processing of formulations (including the aforementioned lamellar phases). The SANS experiment starts by generating a spatial microchannel map by fine xy neutron transmission scans, illustrated in figure 1(b, d, e), followed by the spatiotemporal mapping at selected positions. Two-dimensional SANS acquisitions as short as 1 s are able to resolve the spatial flow response of these complex fluids at the molecular level.
(a) Schematic of a microfluidic-SANS experiment, depicting patterned microchannel geometries and an illustration of a lamellar system, microdevice mounted on a motorised xyz stage for spatio-temporal mapping and 2D scattering pattern. (b) Transmission mapping of the microfluidic device with a 500 μm diameter neutron beam along the microchannel length, with ( ) 10 s and (
) 10 s and ( ) 60 s acquisitions. (c) Optical micrograph of a 6-constriction extensional flow device. (d) Lateral scans (y axis) along a wide (C–D) channel and a constriction (A–B). The grey line is the computed normalised transmitted intensity from the beam and microchannel overlap.
) 60 s acquisitions. (c) Optical micrograph of a 6-constriction extensional flow device. (d) Lateral scans (y axis) along a wide (C–D) channel and a constriction (A–B). The grey line is the computed normalised transmitted intensity from the beam and microchannel overlap.
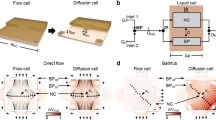
Figure 2a-d examines the flow response of the CTAC/pentanol/D2O lamellar system flowing through a contraction-expansion geometry with flow rates between 0 and 10 mL/hr, corresponding to average velocities of up to 50 mms−1. The schematic of the first constriction and selected reference points (entry, constriction and exit) are indicated, along with corresponding 2D scattering profiles, radial and azimuthal averages. As reported earlier25, we find that the fluid orients parallel to the flow direction (illustrated by ‘blue’ lamellae on the schematic), at the entry and within the constriction and then perpendicularly (‘green’ lamellae) upon exiting the constriction, as it decelerates and experiences an orthogonal extension. This applies to all constrictions in series and at all flow rates examined (including the ‘at rest’ condition, corresponding to a fluid introduced at low flow rate (0.5 mL/hr) and allowed to relax for at least 15 min). The radially-averaged scattering profiles are well modelled by a Lorentzian function I(q) = A/(1 + (q − q0)/S)2 + B, where A is the height of the peak, q0 the peak position, S the full width half maximum (FWHM) and B the incoherent background, in the q-range 0.07–0.2 Å−1. The integrated scattering intensity and peak sharpness increase as the system flows along the channel. Specifically, the intensity increases by approximately 50% upon entering the constriction and by an additional 60% upon exiting. As illustrated in Fig. 2a, we interpret these results as arising from a lamellar sheet rotation from the xz to yz plane, thereby increasing the coherent scattering intensity, as well as from xy to the yz plane, thus flipping the angle of the structural peak. The radially averaged profiles at the entry and constriction remain unchanged with increasing flow rate. At the exit, however, the scattering intensity nearly doubles compared to the ‘rest’ reference and an additional shoulder appears at  , where q* is the position of the main peak, which is not present at other positions and indicates strong lamellar structural alignment normal to the flow direction.
, where q* is the position of the main peak, which is not present at other positions and indicates strong lamellar structural alignment normal to the flow direction.
CTAC/pentanol/D2O under microflow.
(a) Schematic of a microchannel constriction. Green, blue and red sheets represent lamellae orientations in the yz, xy and xz planes as indicated. Radially (left) and azimuthally (right) averaged profiles for positions Ent, Const and Exit, indicated on the schematic. Black, red, green and blue symbols correspond to, respectively, rest, 2, 5 and 10 mL/hr flow rates. (b) 2D scattering profiles at the constriction as a function of flow rate and enlarged profile for 10 mL/hr, including azimuthal sectors, analysed in (c) and (d). (c) Representative I(q) profiles for sectors 0, 2π/3 and 3π/2; lines are fits to a Lorentzian profile I(q) = A/(1+(q − q0)/S)2 + B. d) Fitting parameters A, B, q0, S as a function of azimuthal angle for 10 mL/hr. e) (i) Schematic of a wedge device, where red arrow indicates flow direction; (ii) transmission of the empty cell (grey  , T ≈ 0.98) and sample-filled channel at rest (red
, T ≈ 0.98) and sample-filled channel at rest (red  ); sample transmission follows T = 1.03e−0.463h with height h [mm] and this trend is is validated for h → 0 and
); sample transmission follows T = 1.03e−0.463h with height h [mm] and this trend is is validated for h → 0 and  for a sample in 1 mm Hellma cell standard. (iii) Peak angle in the azimuthal average, indicating the main orientation of the lamellae perpendicular to the xz plane; the blue lines (0, π) correspond to lamellae oriented in the xy plane and the green line (π/2) corresponds to lamellae oriented in the yz plane; (iv) Intensity ratio, referenced to the thickest cross section i.e. (
for a sample in 1 mm Hellma cell standard. (iii) Peak angle in the azimuthal average, indicating the main orientation of the lamellae perpendicular to the xz plane; the blue lines (0, π) correspond to lamellae oriented in the xy plane and the green line (π/2) corresponds to lamellae oriented in the yz plane; (iv) Intensity ratio, referenced to the thickest cross section i.e. ( ) where t is the thickness and T is the transmission and the subscript thick refers to the 0.9 mm point. The scale factor is thus normalised to 1 at t = 0.9 mm.
) where t is the thickness and T is the transmission and the subscript thick refers to the 0.9 mm point. The scale factor is thus normalised to 1 at t = 0.9 mm.
At the constriction entrance, scattering anisotropy increases with increasing flow rate (detailed in Supplementary information Fig S5), as expected for lamellae alignment along the velocity gradient imposed by the constriction. Unexpectedly, however, the azimuthal average corresponding to the system at ‘rest’ within the constriction indicates a larger alignment than that under flow, which we associate with the longer residence time experienced. At the exit, the systems under flow show an even greater degree of anisotropy, but now perpendicularly to the flow direction, as the lamellae sheets decelerate and rotate upon exiting the constriction (the slight tilt in the ‘rest’ pattern is likely due to flow inhomogeneity in these viscous systems). Next we average the 2D scattering profile within the constriction at 10 mL/hr along 12 sectors to obtain I(q) profiles as a function of azimuthal angle and thus orientation under flow. The angular dependence of the Lorentzian fit parameters is shown in figure 2d. Trivially, the peak height A follows the azimuthal profile and we find that its position q0 remains unchanged, as does the scattering background B, within experimental uncertainty. However, along the flow direction, as the peak height A increases, its width decreases, quantifying the lamellar alignment upon lateral compression and acceleration within the constriction. At the exit position, at 10 mL/hr, approximately 50% of the scattering intensity lies between ±10° of the flow direction, indicating strong lamellar alignment.
Our FPP approach is also capable of patterning orthogonally to the microchannel direction45,48 and we next consider a wedge-shaped, or height gradient, microchannel, confined between two quartz windows, depicted in figure 2e (i). Sample injection occurred from the taller towards the thinner channel crossection at constant flow rate 0.5 mL/hr, thus at increasing flow velocity and the SANS measurements reported were carried out at 10 mL/hr. Figure 2e (ii) shows the transmissions, measured at rest, of the empty and sample-filled channel. The former is found to be constant, at T = 0.98, in good agreement with the estimation based on a single quartz plate with a full beam T = 0.992 = 0.98 and characteristic of an outstanding neutron cell. The sample transmission in the microchannel decreases exponentially with height h, as expected and validated for h → 0 and independent control measurements, allowing precise data calibration and computation of the 3D geometry. Figure 2e (iii) plots the peak angle in the azimuthal average corresponding to the main orientation of the lamellae contributing to coherent scattering (i.e. parallel to the neutron beam). A change from orthogonal alignment with respect to the flow direction at the channel entrance, towards parallel alignment at the thinner section is observed. The scattering intensity, after appropriate thickness normalisation, is found to decrease towards the thinner section of the microchannel wedge, along the velocity and extension gradient, quantifying the relative orientational populations. The intensity ratio, referenced with respect to the thick cross-section, is shown in figure 2e (iv). This decrease accompanies the flow alignment of the lamellae sheets along the velocity direction (and thus perpendicularly to the neutron beam, indicated by ‘green’ to ‘red’ lamellae in the schematic), in agreement with the flow alignment parallel to the narrower channel walls found above.
We next investigate an SDS/octanol/d-brine mixture, to evaluate whether similar lamellar rotations, as observed in the CTAC system, are present in multilamellar vesicle (MLV) forming systems17,18. The characteristic inter-lamellar spacing of MLVs is now ≈150 Å, scattering is generally isotropic and we re-configure the spectrometer to a lower q range (and lower neutron flux). We first report on a cross slot geometry, depicted in figure 3a, generating an extensional flow around the central stagnation point. A representative 2D scattering profile at flow rate 0.3 mL/hr is shown and radially-averaged scattering and peak intensities as a function of flow rates (0.3–60 mL/hr) are computed. The decrease in scattering intensity with flow quantifies the disruption of lamellar ordering by flow, but the insensitivity of the peak position q* with flow rate, within experimental uncertainty, indicates that the lamellar spacing remains unchanged.
SDS/octanol/d-brine system under microflow.
(a) Cross slot device schematic and 2D scattering profile for total flow rate of 0.3 mL/hr; bottom left shows representative radial averages as a function of flow rate and bottom right plots peak intensity I(q*) as a function of flow rate. (b) Contraction-expansion series schematic and 2D scattering patterns at flow rates 1 (black), 5 (red), 10 (blue symbols) mL/hr; the mean velocities in the wide channel section (v) are shown in the left column with corresponding azimuthal and radial averages. (c) 2D scattering profiles at Entry and Exit5 after cessation of flow, following 0.5 mL/hr, for Δt = 15 and 30 min.
The SDS system's response through a series of constrictions is evaluated in figure 3b, showing 2D scattering patterns (positions marked in the schematic) at flow rates of 1, 5 and 10 mL/hr and average flow velocities indicated. The system is aligned from the outset, following injection into the device. At the entry position, the system is least aligned at the lowest flow rate of 1 mL/hr. By contrast, upon expansion (Exit5), the least alignment is found at 10 mL/hr, indicating a correlation between alignment and residence time within the device, rather than flow magnitude. An orientational flip at the constriction exit is observed, as found for the CTAC system. The peak positions q* do not vary with flow rate, like in the cross slot geometry, indicating no change of lamellar spacing in this range of flow type and residence time. The overall intensities at 1 mL/hr increase by a factor of 1.5 from the entry position to the constriction and by a further 40% from the constriction to the fifth expansion. At the entry and constriction, the intensities are not significantly affected by flow rate, while at the expansion they decrease by 30% when the flow rate is increased from 1 to 10 mL/hr. Figure 3c considers the structural relaxation of the system over time following cessation of flow after steady state 0.5 mL/hr conditions. Two time points are shown, Δt = 15 and 30 min, enabling the quantification of the relaxation processes both in terms of lamellar orientation and structure.
We have thus resolved the lamellar rotations and alignment experienced by two distinct, representative lamellae-forming systems under different flow types and magnitudes. For the case of contraction-expansion flows, we find that both systems align parallel to the flow before and within the constriction and perpendicularly upon exit, which appear to be general phenomena for anisotropic particle suspensions31,32. We also find that significant rotation of lamellae from the plane perpendicular to the neutron beam to the planes parallel to the neutron beam occurs both from before to within the extensional fields (induced by constrictions) and further upon exit. Thus, both types of rotations appear to be universal to systems with significantly different molecular structures. From a measurement perspective, we demonstrate 2D SANS data acquisition times down to 1 s, while longer acquisition times (≈5 min from 100 μm channels) yield sufficient statistics for quantitative azimuthal sector analysis.
Discussion
In this work, we have demonstrated the coupling of microfluidics and SANS, scattering from single microchannels  wide. Our microdevices are fabricated using a fast and inexpensive rapid prototyping FPP method, which permits both lateral and orthogonal patterning, as required for precise flow field control. Device fabrication takes less than 1 hr and does not require clean-room facilities or complex equipment.
wide. Our microdevices are fabricated using a fast and inexpensive rapid prototyping FPP method, which permits both lateral and orthogonal patterning, as required for precise flow field control. Device fabrication takes less than 1 hr and does not require clean-room facilities or complex equipment.
We acknowledge that concentrated surfactant systems form highly scattering, ordered liquid crystalline phases and that the general suitability of our approach must be further investigated. Based on current SANS instrumentation performance and typical scattering behaviour of model systems, we next establish a ‘capability map’ for microfluidic-SANS. We illustrate in Fig. 4 the scattering features of representative soft matter systems, namely the relevant scattering vector ranges, characteristic scattering intensities (in absolute units cm−1) and typical corresponding neutron flux. We focus on the interplay between experimental parameters from a user perspective and estimate the required acquisition times for a reference microfluidic channel of 500 μm depth and a 500 μm diameter neutron beam, viz. a volume of  (acquisition times for 100 μm × 100 μm channels would simply read ×25). We populate the graph with systems ranging from weakly scattering dilute polymer solutions, which also require relatively low q measurements, to strongly-scattering microemulsion and crystalline systems, generally measured at higher q, for which neutron flux is highest. We consider systems requiring less than 1 min acquisition times, to readily enable fine mapping with sub-mm beam sizes, as well as high-throughput experiments and are thus shown in ‘green’. Other systems, while compatible with current SANS capabilities, are limited in the number of acquisitions possible during a standard SANS experiment, which have a typical duration 24–72 hr. Systems whose required acquisition times are greater than 100 min are considered unfeasible at present (indicated ‘red’ in the graph) and higher flux neutron sources will be required to explore these with microfluidic-SANS. For reference, the mounting and mapping of devices in this work was typically achieved within one hour and measurement times per state point ranged from a few seconds to a few minutes. The time required to acquire all data presented in this paper, including setup, device mounting, mapping and equilibration times, was approximately 24 hr, thereby establishing the feasibility of microfluidic-SANS experiments within a typical beam time allocation.
(acquisition times for 100 μm × 100 μm channels would simply read ×25). We populate the graph with systems ranging from weakly scattering dilute polymer solutions, which also require relatively low q measurements, to strongly-scattering microemulsion and crystalline systems, generally measured at higher q, for which neutron flux is highest. We consider systems requiring less than 1 min acquisition times, to readily enable fine mapping with sub-mm beam sizes, as well as high-throughput experiments and are thus shown in ‘green’. Other systems, while compatible with current SANS capabilities, are limited in the number of acquisitions possible during a standard SANS experiment, which have a typical duration 24–72 hr. Systems whose required acquisition times are greater than 100 min are considered unfeasible at present (indicated ‘red’ in the graph) and higher flux neutron sources will be required to explore these with microfluidic-SANS. For reference, the mounting and mapping of devices in this work was typically achieved within one hour and measurement times per state point ranged from a few seconds to a few minutes. The time required to acquire all data presented in this paper, including setup, device mounting, mapping and equilibration times, was approximately 24 hr, thereby establishing the feasibility of microfluidic-SANS experiments within a typical beam time allocation.
Microfluidic-SANS roadmap, illustrating acquisition times for representative soft matter systems, characterised by typical absolute intensity (cm−1), q-range and configuration required, referenced to a volume of 0.1 μl.
Solid lines correspond to representative SANS configu-rations and fluxes for different q ranges: high q (0.05–0.5 Å−1), mid q (0.01–0.2 Å−1), mid/low q (0.005–0.05 Å−1); dashed lines indicate the expected neutron flux increases over the next decade. 1: Dilute carbon nanotube dispersion, 2: Dilute polymer solution, 3: Dilute surfactant solution, 4: Semidilute/concentrated polymer solution, 5: Polymer solution or simple liquid mixture near phase boundary, 6: Concentrated surfactant solution, 7: L3 phase microemulsion, 8: Multilamellar vesicle emulsion (e.g., SDS system studied in this paper), 9: Lα phase (e.g. the CTAC system studied in this paper), 10: Cubic surfactant phase. We colour-code the background from readily feasible (green) to currently unfeasible microfluidic-SANS experiments (red).
Continued neutron flux improvements will enlarge the range of systems amenable to microfluidic-SANS studies within feasible timescales, indicated by the dashed lines in Fig. 4. The prospect of third generation sources (including the ESS in Sweden50) and high performance time-of-flight SANS spectrometers will contribute to further expand the feasibility of microfluidic-SANS. Predicted gains of order 10–100 in flux and wider scattering vector ranges (qmax/qmin) of 3–4 orders of magnitude will allow the interrogation of increasingly small sample volumes and lower sample contrasts. Smaller beam diameters, down to 10 μm, as well as non circular geometries may thus be employed for finer spatial mapping. Throughout this experiment only one (of 3 possible) scattering planes was investigated; through multilevel patterning and device rocking, all flow directions may be accessible. Further to rheological studies of flow-responsive systems, the high-throughput screening of mixtures, in continuous and droplet flows, is expected to become a reality within the near future.
Methods
Microdevice fabrication
The microfluidic devices were fabricated following a modified frontal photo polymerisation (FPP) approach45,48. In short, a thiol-ene copolymer is UV-A polymerised between optically transparent surfaces, through a photomask defining the lateral geometry and unpolymerised regions are developed by a selective solvent, resulting in the microchannels. In order to minimise scattering background and increase transmission, thin borosilicate or quartz cover slides are employed, with thickness of 140 and 500 μm respectively. Fluid inlets and outlets are drilled through the glass surfaces and capped with connectors for tubing connected to syringe pumps. Thin (~100 μm) microchips are excessively brittle and prone to failure during port drilling and device operation and are therefore mechanically reinforced with a 1 mm thick glass slide with a pre-drilled window around the active microchannels. For this paper, we fabricate passive flow geometries including a rectangular channel with a series of six constrictions, a cross-slot and a tapered channel, achieving lateral dimensions as small as 60 μm and effectively with no upper limit ( ).
).
SANS setup
The SANS experiments were carried out at the D22 spectrometer of the Institut Laue Langevin, Grenoble, France, with an incident wavelength λ = 6 Å, sample-detector distances (SDD) of 2 m and 5.6 m, collimation 2.8 and 5.6 m, yielding a scattering vector range q of 0.025–0.33 and 0.01–0.13 Å−1 respectively, where elastic scattering vector  and θ is the scattering angle (further detailed in Supplementary Information). Circular cadmium diaphragms restricted the beam diameter to 500 or 1000 μm. The results were normalised and calibrated according to standard procedures (GRASP v6.89). The microdevices were mounted on an upright motorised pedestal with xyz motion and Braintree BS-8000 and Harvard PHD2000 syringe pumps were controlled via LabVIEW.
and θ is the scattering angle (further detailed in Supplementary Information). Circular cadmium diaphragms restricted the beam diameter to 500 or 1000 μm. The results were normalised and calibrated according to standard procedures (GRASP v6.89). The microdevices were mounted on an upright motorised pedestal with xyz motion and Braintree BS-8000 and Harvard PHD2000 syringe pumps were controlled via LabVIEW.
Neutron mapping of microdevices
The experimental setup is illustrated in figure 1(a) and an optical micrograph of a 6-constriction microdevice is shown in (c), with depth d = 540 μm, width of wide channel W = 2000 μm and constriction widths  . Microchannel mapping is achieved in two stages: a coarse grid map is first achieved by a red laser alignment along the main features, followed by neutron transmission scans of the empty chips along the x and y directions. Representative vertical (y) scans, across wide and narrow channels, are shown in figure 1d; an x-axis scan along the microchannel centreline is shown in figure 1b. The hydrogenous thiol-ene matrix has a 6Å neutron transmission
. Microchannel mapping is achieved in two stages: a coarse grid map is first achieved by a red laser alignment along the main features, followed by neutron transmission scans of the empty chips along the x and y directions. Representative vertical (y) scans, across wide and narrow channels, are shown in figure 1d; an x-axis scan along the microchannel centreline is shown in figure 1b. The hydrogenous thiol-ene matrix has a 6Å neutron transmission  , which is sufficient to locate microchannels with 5 s transmission measurements. The microchannel geometry, approximately known from optical microscopy, is directly obtained from precise neutron transmission mapping and subsequently employed in data reduction and calibration.
, which is sufficient to locate microchannels with 5 s transmission measurements. The microchannel geometry, approximately known from optical microscopy, is directly obtained from precise neutron transmission mapping and subsequently employed in data reduction and calibration.
Surfactant mixtures
We select two concentrated surfactant mixtures to evaluate the feasibility and potential of microfluidic-SANS. The first is a CTAC/pentanol/D2O mixture at ratios 17.4/62.6/20 wt%, which forms an Lα phase with excess water and interlamellar distance  25. Scattering profiles were acquired using a 500 μm diameter beam for 3 min for the positions in the wider channel and for 6 min in the 100 μm constriction. The beam footprint thus over-illuminates the latter and the acquired signal comprises also device background scattering, which is corrected. Such measurements yield good statistics (~105 counts) in the full q range, however 1 s acquisitions suffice to resolve the peak position (detailed in Supplementary information). The second system investigated is an SDS/octanol/d-brine multi-lamellar vesicle (MLV) forming mixture, which has been studied extensively by Roux and co-workers17,18 by small angle light scattering and microscopy. As the interlamellar peak occurs at lower q, we use a 5.6 m SDD to study this system. Scattering profiles were acquired using a 1 mm diameter beam for 5 minutes (corresponding to ~105 counts) for the positions in the main channel and for 10 minutes in the constriction. Data acquisition of 10 s yields reasonable statistics for the full q range and permits azimuthal averaging (detailed in Supplementary information). All samples were injected into the chip at 0.5 mL/hr and allowed to rest for 15 minutes and are referred to ‘at rest’ throughout the paper, although the systems are generally not completely relaxed from the outset.
25. Scattering profiles were acquired using a 500 μm diameter beam for 3 min for the positions in the wider channel and for 6 min in the 100 μm constriction. The beam footprint thus over-illuminates the latter and the acquired signal comprises also device background scattering, which is corrected. Such measurements yield good statistics (~105 counts) in the full q range, however 1 s acquisitions suffice to resolve the peak position (detailed in Supplementary information). The second system investigated is an SDS/octanol/d-brine multi-lamellar vesicle (MLV) forming mixture, which has been studied extensively by Roux and co-workers17,18 by small angle light scattering and microscopy. As the interlamellar peak occurs at lower q, we use a 5.6 m SDD to study this system. Scattering profiles were acquired using a 1 mm diameter beam for 5 minutes (corresponding to ~105 counts) for the positions in the main channel and for 10 minutes in the constriction. Data acquisition of 10 s yields reasonable statistics for the full q range and permits azimuthal averaging (detailed in Supplementary information). All samples were injected into the chip at 0.5 mL/hr and allowed to rest for 15 minutes and are referred to ‘at rest’ throughout the paper, although the systems are generally not completely relaxed from the outset.
References
Whitesides, G. M. The origins and the future of microfluidics. Nature 442, 368–373 (2006).
Duffy, D. C., Mcdonald, J. C., Schueller, O. J. A. & Whitesides, G. M. Rapid Prototyping of Microfluidic Systems in Poly (dimethylsiloxane). Anal. Chem. 70, 4974–4984 (1998).
Sollier, E., Murray, C., Maoddi, P. & Di Carlo, D. Rapid prototyping polymers for microfluidic devices and high pressure injections. Lab Chip 11, 3752–3765. (2011).
Stone, H., Stroock, A. & Ajdari, A. Engineering of flow in small devices. Annu. Rev. Fluid Mech. 36, 381–411 (2004).
Squires, T. M. & Quake, S. R. Microfluidics: Fluid physics at the nanoliter scale. Rev. Mod. Phys. 77 977 (2005).
Song, H., Chen, D. L. & Ismagilov, R. F. Reactions in droplets in microfluidic channels. Angew. Chemie - Int. Ed. 45, 7336–7356 (2006).
Lee, C.-Y., Chang, C.-L., Wang, Y.-N. & Fu, L.-M. Microfluidic mixing: a review. Int. J. Mol. Sci. 12, 3263–3287 (2011).
Capretto, L., Cheng, W., Hill, M. & Zhang, X. Micromixing within microfluidic devices. Top. Curr. Chem. 304, 27–68 (2011).
Li, L. & Ismagilov, R. F. Protein crystallization using microfluidic technologies based on valves, droplets and SlipChip. Annu. Rev. Biophys. 39, 139–158 (2010).
Hansen, C. & Quake, S. R. Microfluidics in structural biology: Smaller, faster… better. Curr. Opin. Struct. Biol. 13, 538–544 (2003).
Pipe, C. J. & McKinley, G. H. Microfluidic rheometry. Mech. Res. Commun. 36, 110–120 (2009).
Rodd, L. E., Cooper-White, J. J., Boger, D. V. & McKinley, G. H. Role of the elasticity number in the entry flow of dilute polymer solutions in micro-fabricated contraction geometries. J. Nonnewton. Fluid Mech. 143, 170–191 (2007).
Clasen, C. & Kulicke, W.-M. Determination of viscoelastic and rheo-optical material functions of water-soluble cellulose derivatives. Prog. Polym. Sci. 26, 1839–1919 (2001).
Medronho, B. et al. Shear-induced transitions between a planar lamellar phase and multilamellar vesicles: Continuous versus discontinuous transformation. Langmuir 24, 6480–6486 (2008).
Muller, S., Borschig, C., Gronski, W., Schmidt, C. & Roux, D. Shear-induced states of orientation of the lamellar phase of C12E4/water. Langmuir 15, 7558–7564 (1999).
Larson, R. G. Flow-induced mixing, demixing and phase transitions in polymeric fluids. Rheol. Acta 31, 497–520 (1992).
Sierro, P. & Roux, D. Structure of a Lyotropic Lamellar Phase under Shear. Phys. Rev. Lett. 78, 1496–1499 (1997).
Wunenburger, A. S., Colin, A., Leng, J., Arneodo, A. & Roux, D. Oscillating viscosity in a lyotropic lamellar phase under shear flow. Phys. Rev. Lett. 86, 1374–1377 (2001).
de Gennes, P. G. Scaling Concepts in Polymer Physics (Cornell University Press: London., 1979).
Soft-Matter Characterization [Borsali, R. & Pecora, R. (eds.)] (Springer, 2008).
Sato, J. & Breedveld, V. Transient rheology of solvent-responsive complex fluids by integrating microrheology and microfluidics. J. Rheol. (N. Y. N. Y). 50, 1 (2006).
Kazarian, S. G. Enhancing high-throughput technology and microfluidics with FTIR spectroscopic imaging. Anal. Bioanal. Chem. 388, 529–532 (2007).
Akiyama, S. et al. Conformational landscape of cytochrome c folding studied by microsecond-resolved small-angle x-ray scattering. Proc. Natl. Acad. Sci. U. S. A. 99, 1329–34 (2002).
Russell, R. et al. Rapid compaction during RNA folding. Proc. Natl. Acad. Sci. U. S. A. 99, 4266–71 (2002).
Martin, H. Microfluidic processing and in-situ analysis of model concentrated surfactant solutions. Ph.D. thesis, Imperial College London. (2011).
Kathuria, S. V. et al. Minireview: Structural insights into early folding events using continuous-flow time-resolved small-angle X-ray scattering. Biopolymers 95, 550–558 (2011).
Reich, C., Hochrein, M. B., Krause, B. & Nickel, B. A microfluidic setup for studies of solid-liquid interfaces using x-ray reflectivity and fluorescence microscopy. Rev. Sci. Instrum. 76, 095103 (2005).
Körstgens, V. et al. Following initial changes in nanoparticle films under laminar flow conditions with in situ GISAXS microfluidics. RSC Adv. 4, 1476–1479 (2014).
Lafleur, J. P. et al. Automated microfluidic sample-preparation platform for high-throughput structural investigation of proteins by small-angle X-ray scattering. J. Appl. Crystallogr. 44, 1090–1099 (2011).
Merlin, A. et al. Time-resolved microfocused small-angle X-ray scattering investigation of the microfluidic concentration of charged nanoparticles. Eur. Phys. J. E. Soft Matter 34, 1–7 (2011).
Dootz, R., Evans, H., Köster, S. & Pfohl, T. Rapid prototyping of X-ray microdiffraction compatible continuous microflow foils. Small 3, 96–100 (2007).
Trebbin, M. et al. Anisotropic particles align perpendicular to the flow direction in narrow microchannels. Proc. Natl. Acad. Sci. U. S. A. 110, 6706–6711 (2013).
Higgins, J. S. & Benoit, H. C. Polymers and Neutron Scattering (Oxford University Press., 1994).
Benoit, H. et al. Dimensions of a Flexible Polymer Chain in the Bulk and in Solution. Nature 245, 13–15 (1973).
Schleger, P., Farago, B., Lartigue, C., Kollmar, A. & Richter, D. Clear Evidence of Reptation in Polyethylene from Neutron Spin-Echo Spectroscopy. Phys. Rev. Lett. 81, 124–127 (1998).
Willner, L., Poppe, A., Allgaier, J., Monkenbusch, M. & Richter, D. Time-resolved SANS for the determination of unimer exchange kinetics in block copolymer micelles. Europhys. Lett. 55, 667–673 (2007).
Roe, R.-J. Methods of X-ray and Neutron Scattering in Polymer Science (Oxford University Press., 2000).
Eberle, A. P. & Porcar, L. Flow-SANS and Rheo-SANS applied to soft matter. Curr. Opin. Colloid Interface Sci. 17, 33–43 (2012).
Grillo, I. Applications of stopped-flow in SAXS and SANS. Curr. Opin. Colloid Interface Sci. 14, 402–408 (2009).
Cloke, V. M. et al. Poiseuille geometry shear flow apparatus for small-angle scattering experiments. Rev. Sci. Instrum. 67, 3158 (1996).
Penfold, J. et al. Elongational flow induced ordering in surfactant micelles and mesophases. J. Phys. Chem. B 110, 1073–1082 (2006).
Lutz-Bueno, V., Kohlbrecher, J. & Fischer, P. Micellar solutions in contraction slit-flow: alignment mapped by SANS. J. Non-Newt. Fluid Mech. 211, 31–49 (2014).
Bent, J. et al. Neutron-mapping polymer flow: scattering, flow visualization and molecular theory. Science 301, 1691–1695 (2003).
Zhao, Y., Cheung, P. & Shen, A. Q. Microfluidic flows of wormlike micellar solutions. Adv. Colloid Interface Sci. 211, 34–46 (2014).
Cabral, J. T., Hudson, S. D., Harrison, C. & Douglas, J. F. Frontal photopolymerization for microfluidic applications. Langmuir 20, 10020–10029 (2004).
Sollier, E., Murray, C., Maoddi, P. & Di Carlo, D. Rapid prototyping polymers for microfluidic devices and high pressure injections. Lab on a Chip 11, 3752–3765 (2011).
Bartolo, D., Degré, G., Nghe, P. & Studer, V. Microfluidic stickers. Lab on a Chip 8, 274–279 (2008).
Harrison, C., Cabral, J. T., Stafford, C. M., Karim, A. & Amis, E. J. A rapid prototyping technique for the fabrication of solvent-resistant structures. J. Micromech. Microeng. 14, 153–158 (2003).
Rosen, M. J. & Kunjappu, J. T. Surfactants and Interfacial Phenomena (John Wiley & Sons., 2012).
Argyriou, D. N. Reinventing neutron science in europe. Nature Mater 13, 767–768 (2014).
Acknowledgements
We thank the Institut Laue Langevin for neutron beamtime, EPSRC and Unilever for a CASE award for CG.L. and Erasmus Mundus for a visiting scholarship for T.W. We acknowledge J. Davies for assistance in microdevice fabrication, K. Honnibal (ILL) for technical drawings, H.P. Martin (Imperial) for help in formulating the model systems and P. Graham and A. Kowalski (Unilever) for discussions.
Author information
Authors and Affiliations
Contributions
Microdevice fabrication was carried out by T.W. and C.G.L. Spectrometer configuration and optimisation was carried out by L.P. and A.M. C.G.L. performed the SANS data analysis. C.G.L., L.P. and J.T.C. planned and designed the experiment and prepared the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Lopez, C., Watanabe, T., Martel, A. et al. Microfluidic-SANS: flow processing of complex fluids. Sci Rep 5, 7727 (2015). https://doi.org/10.1038/srep07727
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07727
This article is cited by
-
Scattering-Informed Microstructure Prediction during Lagrangian Evolution (SIMPLE)—a data-driven framework for modeling complex fluids in flow
Rheologica Acta (2023)
-
Small-angle X-ray and neutron scattering
Nature Reviews Methods Primers (2021)
-
Probing flow-induced nanostructure of complex fluids in arbitrary 2D flows using a fluidic four-roll mill (FFoRM)
Scientific Reports (2018)
-
A Small-Angle Neutron Scattering Environment for In-Situ Observation of Chemical Processes
Scientific Reports (2018)
-
The role of small-angle scattering in structure-based screening applications
Biophysical Reviews (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.