Abstract
Animal waste from concentrated swine farms is widely considered to be a source of environmental pollution and the introduction of veterinary antibiotics in animal manure to ecosystems is rapidly becoming a major public health concern. A housefly larvae (Musca domestica) vermireactor has been increasingly adopted for swine manure value-added bioconversion and pollution control, but few studies have investigated its efficiency on antibiotic attenuation during manure vermicomposting. In this study we explored the capacity and related attenuation mechanisms of antibiotic degradation and its linkage with waste reduction by field sampling during a typical cycle (6 days) of full-scale larvae manure vermicomposting. Nine antibiotics were dramatically removed during the 6-day vermicomposting process, including tetracyclines, sulfonamides and fluoroquinolones. Of these, oxytetracycline and ciprofloxacin exhibited the greater reduction rate of 23.8 and 32.9 mg m−2, respectively. Environmental temperature, pH and total phosphorus were negatively linked to the level of residual antibiotics, while organic matter, total Kjeldahl nitrogen, microbial respiration intensity and moisture exhibited a positive effect. Pyrosequencing data revealed that the dominant phyla related to Firmicutes, Bacteroidetes and Proteobacteria accelerated manure biodegradation likely through enzyme catalytic reactions, which may enhance antibiotic attenuation during vermicomposting.
Similar content being viewed by others
Introduction
Large quantities of raw manure are produced in swine farms throughout the world today as a result of the development of concentrated animal feeding operations1. Animal manure is traditionally applied to farmlands as a bio-fertilizer to provide essential nutrients, which may cause environmental pollution due to the release of pathogens and excess nutrients into bodies of water resulting in the public health risk of eutrophication2. Best management practices, such as composting and anaerobic digestion, have been proposed for animal waste prior to land application in order to mitigate health and environmental risks2; however, these options cannot be broadly applied, particularly in developing countries, due to limitations in efficiency and cost. Thus, there is an urgent need to develop both economically and environmentally sustainable technologies for swine manure management.
Some studies have exploited the ability of several species of diptera to grow naturally on animal waste as a substrate3,4,5,6 and diptera larvae have been used instead of earthworms3,4,7,8 to vermicompost animal and/or agroindustrial waste. In particular, the housefly (Musca domestica) was chosen for vermicomposting of animal manure7,9 due to its short interval of generation (5–7 days) and high reproductive capacity1,5,7. While previous studies have validated the overall process and economic feasibility of housefly larvae-vermicomposting1,5,7, little is known about its efficiency in the degradation and removal of contaminants, such as veterinary antibiotics, in animal manure.
Current livestock production uses large amounts of antibiotics as growth promoters and therapeutic agents10. Tetracyclines (TCs), sulfonamides (SAs) and quinolones (QLs) are three classes of broad-spectrum antibiotics widely used in the livestock industry in China11,12. It is estimated that approximately 75% of the antibiotics used are not absorbed and are instead excreted in waste10. Antibiotic residue in manure can reach the soil-water ecosystem by run-off and/or leaching, creating one of the main sources of terrestrial pharmaceutical pollution and is of major concern to environmental and human health13,14,15. A number of studies have demonstrated that incubating animal manure under a variety of composting conditions results in a significant decrease in the concentration of extractable antibiotics16,17,18, primarily due to abiotic degradation and sorption18. Compared to conventional composting, housefly larvae-vermicomposting has been shown to have a superior biodegradation capacity due to the presence of the unique larvae-microorganism complexes5,7. Therefore, housefly larvae-vermicomposting could be potentially effective in the degradation of veterinary antibiotics in animal manure.
A full-scale operation of swine manure vermicomposting with housefly larvae has been under operation since 2008 in HangZhou, China. In this study, the attenuation of antibiotics as well as biochemical and microbial characteristics of the vermicomposting process was monitored within a typical generation interval (6 days) with the following objectives: 1) assess the effectiveness of housefly larvae-vermicomposting in the degradation of antibiotic residues in swine manure; 2) evaluate the linkages between the degradation of veterinary antibiotics and reduction in manure mass; and 3) gain insight into the degradation processes responsible for antibiotic attenuation.
Results
Dynamics of antibiotic attenuation during housefly larvae vermicomposting
Vermicomposting of swine manure had a significant impact on the concentration of all antibiotics tested, while few changes occurred under the ambient conditions with no larvae inoculation (Table S1). The reduction in two classes of antibiotics, TCs and SAs, was consistent throughout vermicomposting (Fig. 1). Among TCs and SAs, levels of oxytetracycline (OTC), chlortetracycline (CTC) and sulfadiazine (SDZ) exhibited the greatest reduction across the 6-day bioconversion with average rates of 23.8 mg m−2, 8.34 mg m−2 and 4.09 mg m−2 within vermicomposting (Table 1). These values represented cumulative removals of 68.6%, 69.8% and 72.3% of the total antibiotic mass from raw manure input, respectively (Fig. 1). Some rebound in antibiotic content was observed on day 6 (Table S1) due to the addition of manure solids before larvae harvesting, which caused some decrease in the daily reduction rates (Table 1). The trend in the rate reduction across the class of QLs was not consistent, with the exception of ofloxacin (OFC) and averaged 1.17 mg m−2 and 32.9 mg m−2 for norfloxacin (NFC) and ciprofloxacin (CFC), respectively (Table 1), while the cumulative removal reached 34.3–58.1%. All nine antibiotics exhibited rapid reduction during vermicomposting, with daily reduction percentages ranging from 7.8% to 57.4% (Fig. 1). However, the daily rate of reduction for all antibiotics declined over time (Table 1).
Cumulative and daily antibiotic reduction in vermicompost after 1 (T-1), 3 (T-3), 5 (T-5) and 6 days (T-6) during swine manure larvae vermicomposting.
Nine common antibiotics were detected: tetracycline (TC), oxytetracycline (OTC), chlortetracycline (CTC), doxycycline (DOC), sulfadiazine (SDZ), norfloxacin (NFC), ofloxacin (OFC), ciprofloxacin (CFC) and enrofloxacin (EFC).
Dynamics of swine manure reduction during housefly larvae vermicomposting
Under the semi-continuous feeding mode, the content of organic matter (OM) was clearly reduced from 91.4% (dry weight) in raw manure to 83.7% in the vericompost during one-week of vermicomposting (Fig. S1). Moreover, the average content of total Kjeldahl nitrogen (TKN) decreased from 3.52% to 2.61%, while levels of total phosphorus (TP) and total potassium (TK) remained the same with some minor fluctuations over time. A cumulative reduction rate of 35.5 kg m−2 for total manure mass was achieved following the 6-day vermicomposting, leaving 17.8 kg m−2 of vermicompost at the end of the process representing one-third of the total input of raw manure (Fig. 2a). The moisture content of vermicompost dropped continuously from 81.4% to 50.9% (Fig. S2), with a maximum reduction rate of 5.1 kg m−2 d−1 at T-6 (Fig. 2b) and a cumulative reduction rate of 34.3 kg m−2 (Fig. 2a). The pH of the vermicompost increased from 6.67 to 8.46 during vermicomposting with a sharp increase at T-3. In addition, the temperature inside the vermireactor ranged between 30°C and 48°C and increased gradually before day 5 with an apparent drop around the completion of vermicomposting on day 6 (Fig. 2c).
The levels of OM and TKN showed a significant decrease during vermicomposting (p < 0.05), but the levels of TP and TK were not significantly reduced (Fig. 3). The cumulative removal rates for OM, TKN, TP and TK were 4183 g m−2, 211 g m−2, 82 g m−2 and 41 g m−2, respectively (Fig. 3, p < 0.05). The average daily removal rates for OM, TKN, TP and TK followed the same trend, averaging 697 g m−2 d−1, 35 g m−2 d−1, 14 g m−2 d−1 and 7 g m−2 d−1, respectively (Fig. 3, p < 0.05). There were also considerable changes in the biochemical features of the vermicompost (Table 2). The activity of β-1,4-glucosidase (βG) continuously increased, reaching 525 mmol h−1 g−1 at T-5 and then decreased to 369 mmol h−1 g−1 at T-6. Cellobiohydrolase (CBH) activity steadily decreased over time from the highest level of 758 mmol h−1 g−1 at T-1. In addition, housefly larvae caused a significant reduction in the activities of β-1,4-N-acetylglucosaminidase (NAG) and acid phosphatase (AP), which reached 207 mmol h−1 g−1 and 25.9 mmol h−1 g−1 after the 6th day of vermicomposting, respectively (Table 2). A notable increase was observed in the tested enzymes (except βG), with increases in activity by 18% (CBH) to 86% (AP) within the first 3 days compared to control; however, the opposite trend also occurred, with drops by 15% (CBH) to 61% (NAG) at the end of vermicomposting (T-6). In addition, microbial cumulative mineralization was consistently suppressed over the course of vermicomposting, with the respiration rate dropping from 4768 μg g−1 at C-0 to 2016 μg g−1 at T-6 (Fig. 2d).
Response of bacterial diversity during manure larvae vermicomposting
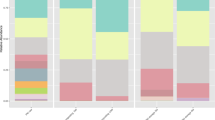
The 16S rRNA 454 sequencing identified bacterial operational taxonomic units (OTUs) from 17 phyla (Firmicutes, Bacteroidetes, unclassified, Proteobacteria, Tenericutes, Spirochaetes, Actinobacteria, Verrucomicrobia, Fibrobacteres, TM7, Synergistetes, Fusobacteria, Lentisphaerae, Cyanobacteria, Chloroplast, OD1, and SR1) in swine manure (C-0) and vermicompost at T-3 and T-6. We selected 9 phyla with the highest relative abundance in order to explore the structural transition of the bacterial community. The predominant populations, which were the Firmicutes, Bacteroidetes and Proteobacteria phyla, changed from 40.5% to 52.9%, 25.9% to 15.6% and 10.0% to 15.7% during vermicomposting, respectively (Fig. 4). The total number of bacterial OTUs also decreased after vermicomposting: Verrucomicrobia, Fibrobacteres and Spirochaetes were absent from vermicompost at T-6, while the regnant phyla Firmicutes, Bacteroidetes and Proteobacteria OTUs declined by 53.9%, 78.7% and 44.9%, respectively (Fig. S3).
The distribution of sequences in the dominant bacterial phyla from raw swine manure (C-0) and vermicompost after 1 (T-1), 3 (T-3), 5 (T-5) and 6 days (T-6).
Relative abundances are based on the proportional frequencies of those DNA sequences that could be classified at the phylum level. The percent values are presented within the respective sections in the columns.
The linkage between antibiotic dynamics and manure/vermicompost features
The relationship between the physico-biochemical properties of vermicompost and the concentration of residual antibiotics was statistically evaluated by redundancy analysis (RDA) (Fig. 5). Axis 1 explained 51.9% of the variance contributed by the differences in characteristics between raw manure and vermicompost, while axis 2 explained 6.9% of the variance from sampling time. Among the environmental and physico-biochemical variables, temperature, pH and TP were negatively linked to the level of residual antibiotics, while OM, TKN, respiration intensity and moisture had a positive effect. Enzymatic activities were not directly linked to antibiotic concentrations. Furthermore, the control and treatment samples were differentiated by RDA (Fig. 6). The Verrucomicrobia, Fibrobacteres and Fusobacteria OTUs were significantly negatively linked to antibiotic levels of vermicompost at T-3 and T-6, including other bacterial phyla. The different characteristics between raw manure and vermicompost contributed to 66.3% of the variance for axis 1, while the variances from sampling time contributed 17.3% to axis 2.
Discussion
In this study, a notable reduction in antibiotic concentration induced by the larvae-microorganism complexes was observed, with cumulative reductions ranging from 34% to 72% for TCs, SAs and QLs (Fig. 1). Previous studies have reported the removal of veterinary antibiotics through traditional composting of animal manure. A 99% reduction in salinomycin concentration was achieved over 38 days14, while more than 99% of NFCs were removed during 40 days of broiler manure composting13. Reductions of 54% to 76% were observed for monensin and tylosin during a period of 22–35 days19. In comparison, the vermicomposting process tested in this study achieved a reduction of up to 70% within 6 days (Table 1, Fig. 1), which is a much shorter time period than that required for traditional composting processes. The shorter duration required for vermicomposting provides a significant advantage of process efficiency for the control of antibiotics in animal manure.
In recent years, many researchers have focused on the attenuation of veterinary antibiotics using various matrix and management technologies. At the environmentally relevant concentrations tested in activated sludge, biodegradation and adsorption were the major removal routes for these antibiotics20,21. Since antibiotics are generally introduced from livestock into the environment, hydrolysis is an important degradation pathway22. Due to the susceptibility to photodegradation, other studies have demonstrated that photolysis is another abiotic transformation process10. In composting, the abiotic degradation conditions, such as temperature and degradation via epimerization, are likely to control the dissipation of antibiotics16,17. In our study, aside from common environmental factors such as temperature and pH (Fig. 5), which positively contribute to antibiotic biodegradation and transformation10,23, the chemical/biochemical factors linked to the reduction of swine manure were also correlated with antibiotic attenuation. Raw manure has abundant OM, TKN and active biological features and contained relative high concentrations of all nine antibiotics. Importantly, it was decomposed with a synchronous antibiotic reduction after vermicomposting using this process (Table S1). Results from this study revealed that the amount of manure reduction was correlated with the removal of antibiotics (Fig. 5) and a remarkable reduction of manure moisture (Fig. 2 and Fig. S1) produced rapid growth and metabolism of aerobic microorganisms23,24,25 due to oxygen enrichment of the vermicompost material. This may be beneficial for antibiotic attenuation and suggests that vermicompost may be a safe approach for use in agriculture. It is noteworthy that QLs had a relatively lower rate of reduction than the other two classes of antibiotics during vermicomposting (Table 1, Fig. 1). There are two possible explanations for this observation. First, the low values of octanol-water partition coefficients (log Kow = 0.61–1.48) and high values of sorption coefficients (Kd = 690–1270, at 28°C) suggest that the adsorption of quinolones from sludge (e.g., electrostatic interaction) is much stronger than hydrophobic interactions21,26. Thus, manure solids and/or vermicompost would be a temporary ‘refuge’ for OL persistence. Second, product analyses have shown that oxidation by MnO2 results in dealkylation and hydroxylation at the piperazine moiety of QLs, with the quinolone ring remaining essentially intact27. This low susceptibility to oxidation could easily maintain active QLs under the anaerobic-prevalent conditions during the process of larvae manure vermicompoting. Therefore, the environmental risk of QLs should be considered over other antibiotics when vermicompost is recycled as a biofertilizer.
The dynamic nature of manure/vermicompost enzymes acts as a medium to control the synchronous reduction of both manure waste and antibiotics, although a direct linkage between enzyme activities and manure antibiotic concentrations was not observed (Fig. 5). The intensities of βG and CBH dynamics have been previously linked to the rate of organic C biodegradation and the maturity of the waste composting process24,25, since the product of CBH is the substrate for the βG catalytic reaction28. This “seesaw”-type co-existence of βG and CBH dynamics from day 1 to day 5 (Table 2) created a synergistic mechanism for the rapid biodegradation of organic C during vermicomposting (Fig. 3). The simultaneous drop in these two enzymes at the end of the vermicomposting process (at day 6) suggested an exhaustion of active organic C pools, leaving the steady and recalcitrant C in vermicompost. Organic N and P were rapidly biodegraded by larvae-microorganism complexes during the first few days of vermicomposting (Fig. 3) with enhanced NAG and AP activities (Table 2), suggesting a nutrient need for microbial metabolism in the vermireactor. The joint enzymatic reactions sped up the consumption of organic matter, accompanied by plentiful nutrients for microbial metabolism, which maintained the exposure of antibiotics to microbial attack. Previous studies have indicated that several antibiotics are hydrolytically susceptible to chemical bonds whose integrity is central to biological activity, such as esters and amides and involves the oxidation–reduction processes due to the formation of an inactive compound29,30. Not surprisingly, all of the enzymes detected in our study are involved in the reactions required to hydrolyze structural glucans, amino acids and phosphates28, which have evolved to target and cleave these vulnerable bonds31. Enzymatic catalysis increases the opportunity for antibiotics to lose their ‘harbor’. Therefore, the force of enzymatic activities could contribute to the positive impacts of OM, TKN, respiration intensity and moisture to antibiotics (Fig. 5), representing a synchronous mechanism for waste reduction and antibiotic attenuation (Table 1, Fig. 3).
Firmicutes and Proteobacteria were the predominant bacterial phyla produced in the vermireactor (Fig. 4, p < 0.05), although the total bacterial population decreased during the process (Fig. S3). There was also a remarkable suppression of microbial respiration (Fig. 2d). Similarly, a study using an earthworm vermireactor fed by agroindustrial lignocellulose wastes confirmed a remarkable decline in bacteria abundance8. The decrease in the abundance of microorganisms could be due to two mechanisms: 1) rapid manure reductions (e.g., moisture, OM and TKN, in Fig. 2 & 3 and Fig. S2) and alterations in physical-chemical features of biomass (e.g., pH, temperature and enzymatic activities, in Fig. 2 and Table 2) that occurred during vermicomposting generated an unfavorable environment for anaerobic-prevailing microorganisms as well as microbes that cannot compete with others for the limited nutrients8,32; and 2) Pathogens cannot survive the gut fluids during the passage of waste through the gut (e.g., earthworms) and are eliminated and/or competitively displaced by the proliferation of other microorganisms32,33. The enhanced proportions of these two phyla demonstrate an abundance of such bacteria to regulate hydrolytic enzyme activities for organic breakdown34,35,36. During the bioconversion process, these serial reactions of microorganism-enzyme-waste directly impacted the degradation of manure/vermicompost antibiotics (Fig. 6). Bacteroidetes exhibited an intimate linkage with antibiotic concentration (Fig. 6), which was largely responsible for the degradation of high molecular weight organic matter, such as proteins and carbohydrates37. During the later stages of vermicomposting, microbial mineralization severely decreased (Fig. 2d) and the bacterial population declined with the absence of three detected phyla (Fig. 4 and Fig. S3). The reduction in Bacteroidetes confirmed the presence of numerous carbohydrate-active enzymes covering a large spectrum of substrates37, which promoted a highly decomposed stage for manure waste and had a positive effect on antibiotic attenuation (Fig. 6). Antibiotics, however, are among the most important groups of pharmaceuticals and chemotherapeutic agents that inhibit or terminate the growth of microorganisms, such as CFC38. The manure, with an abundant bacterial population and concentration of antibiotics (Fig. S3 and Table S1), was stabilized and matured under the vermi-culture created by larvae-microorganism complexes5. The structural transition of the bacterial community (Fig. 4 and Fig. S3) was conducive for accelerating the biodegradation process of antibiotic attenuation (Fig. 6). A similar study reported that the bacterial degradation for doxycycline (DOC) and OTC resulted in a large decrease in more than 50% of the initial antibiotic concentration39, suggesting that the level of bacterial degradation was sufficient to affect the dynamics of antibiotic residue. The response of the bacterial population in manure/vermicompost impacts the waste decomposition process through enzyme catalysis by introducing a readily biodegradable substance, allowing for the monitoring of effects on manure waste and offering an underlying mechanism for antibiotic attenuation at the genetic level.
To the best of our knowledge, this is the first report on the antibiotic removal efficiency using a full-scale, semi-continuous feeding vermireactor. Based on the analysis above, there are two feasible measures that can be taken for technological improvement: 1) an enhancement of feeding manure from day-1 to day-3 for efficiency promotion, since the maximum percentage of daily reduction for antibiotic residues mainly appeared at T-1 and T-3 (Table 1 and Fig. 1); and 2) increasing and maintaining high temperature in the vermireactor, particularly during the winter season and the early stages of vermicomposting, because the higher temperature seems beneficial for antibiotic attenuation (Fig. 5). Although an alkaline condition accelerates the degradation of antibiotics (Fig. 5) and abiotic transformation23, a higher pH inside the vermireactor would cause a greater release of ammonia from the manure residue14,40. Thus, technical caution is needed when commercial alkaline is added to manure during larvae vermicomposting for purposes of improving antibiotic attenuation. Enzyme catalysis and microflora composition may contribute to the removal of swine manure antibiotics via larvae vermireactors. In addition, manure microbiota might be modified, since the gut microbiome of most organisms is dominated by Firmicutes, Bacteroidetes and Proteobacteria41,42, representing more than 1000 different molecular species or phylotypes43. Further research should focus on the functional potential of certain bacteria through combined phylogenetic, taxonomic and metagenomic analyses44 in order to better understand the underlying antibiotic degradation mechanisms. Moreover, the use of veterinary antibiotics may result in the selection of antibiotic-resistant bacteria, which could challenge microbial populations and impact the structure and activity of environmental microbiota14,40. The same resistance genes found in clinical settings are currently disseminated among pristine ecosystems without any record of antibiotic contamination40 due to the high diversity and prevalence of mobile plasmids carrying antibiotic genes in the parent substance14. These may result in a potential hazard on environmental safety and human health18. Due to relatively low biodegradation, the persistence of QLs in animal waste (Fig. 1) could cause serious genetic selection of QL-related resistance genes, since the relative abundance of these genes and the concentrations of QLs were shown to be significantly correlated45. Based on these conclusions, further investigation of whether this full-scale vermireactor can impede resistant bacterial pathogens and plasmid-mediated genes is warranted.
Methods
Design and operation of full-scale larvae vermireactor
In September 2008, a full-scale swine manure vermicomposting operation using housefly (Musca domestica) larvae was installed by the HangZhou TianYuan Agriculture Development Co., Ltd. (30°49′47.02″N, 120°39′22.12″E) located in XiaoShan District, HangZhou, Southeast China. The firm currently possesses 35,000 m2 of greenhouse-assisted larvae vermireactors with a maximum daily treatment capacity of 105 tons per day of fresh raw manure. Raw swine manure (with no solid-liquid separation) was collected from a neighboring swine farm raising approximately 155,000 finishing hogs annually.
The design of the full-scale swine manure vermicomposting facility has been described in detail in previously reports1,5 and can be found in the supplemental material S1. Briefly, four main processing units are involved in the vermicomposting process: 1) seed-fly breeding and fly oviposition, 2) larvae vermicomposting, 3) larvae-vermicompost separation and 4) seed stocking and eclosion (Fig. S4 and Fig. S5). The greenhouse-assisted larvae vermireactors were operated with a semi-continuous feeding mode: 11.0 kg raw manure (moisture content 78–84%, Fig. S2) per m2 was laid as the base feed in each vermireactor at the beginning of vermicomposting; as increasing amounts of manure were consumed by the housefly larvae, new layers of raw manure were added sequentially at the rate of 5.56, 15.6, 15.6 and 5.56 kg m−2 (wet weight) on day-1, day-2, day-3 and day-4, respectively; no additional raw manure was added on day-5 and day-6. The manure in the vermireactor reached an average depth of 8–10 cm at the completion of composting. Manure solids (with moisture of 45%) after solids-liquid separator (sieve opening: 2.5 mm) were added at rate of 6.5 kg m−2 (wet weight) into and mixed with the stack of vermicomposting on day-5, in order to improve the permeability and accelerate the biological dehydration before larvae-vermicompost separation on day 6.
Manure and vermicompost sampling
A batch of raw swine manure was piled under ambient conditions with no larvae addition after 0, 3 and 6 days (Control: C-0, C-3, C-6) and vermicompost (before raw manure feeding) during vermicomposting after 1, 3, 5 and 6 days (Treatment, T-1, T-3, T-5 and T-6, respectively) were collected in triplicates in May 2013. Vermicompost samples (1.0 kg) were taken from four randomly chosen sampling sub-sections (diameter 6 cm) in each vermireactor with a cylindrical stainless–steel container. Temperature and pH in middle layer of manure were recorded for both Control and vermicomposting treatment. After uniform mixed, sub-samples were collected for analysis. All samples were immediately placed in a portable cooler and transported to the laboratory in ZheJiang University within 2 h and stored at −20°C for no more than 8 h before further processing. The samples thereafter were freeze-dried (Labconco, Kansas City, MO) and homogenized by sieving through a 0.15 mm mesh before analysis.
Extraction and analysis of antibiotics
Nine antibiotics, belonging to three classes, frequently detected in environmental samples11,12 were investigated in this study: 1) TCs: tetracycline (TC), oxytetracycline (OTC), chlortetracycline (CTC) and doxycycline (DOC); 2) SAs: sulfadiazine (SDZ); 3) QLs: norfloxacin (NFC), ofloxacin (OFC), ciprofloxacin (CFC) and enrofloxacin (EFC). The concentrations of antibiotics in manure/vermicompost samples were determined by liquid chromatography-tandem mass spectrometry with the isotope-labeled internal standard method as previously described11. Briefly, an aliquot (0.2 g) of each sample was spiked with the surrogate standards and internal standards (100.0 μg kg−1), followed with 20 mL of extraction solvent (EDTA-sodium phosphate buffer with acetonitrile: Mg(NO3)2-NH3·H2O, v/v, 3:1). The extraction mixtures were placed in the dark overnight and centrifuged at 5000 rotations per minute (rpm) for 10 min after rigorous mixing for 30 min at 200 rpmat 25 ± 1°C in the dark and ultrasonication for 15 min. The supernatant containing antibiotics was collected and the same extraction protocol was repeated two more times for the residue. The supernatants from the three extractions were combined, filtered through a 0.7 mm glass microfiber filter (GF/F, Whatman) and diluted to 500 mL with ultrapure water to maintain the organic solvent content <5% in the solution. Then the antibiotics were extracted using ultrasonic-assisted extraction followed by solid phase extraction clean-up with hydrophilic–lipophilic balance cartridges. The antibiotics analytes were separated and detected by liquid chromatography-electrospray ionization tandem mass spectrometry and quantified by the isotope-labeled internal standard method. Mass spectral acquisition was done in the positive ion mode by applying multiple reactions monitoring two fragmentation transitions per analyte to provide a high degree of sensitivity and specificity. The calibration range used for all the antibiotics was 5.0–300 μg L−1.
Chemical analysis
Chemical analysis of manure/vermicompost samples were performed at HangZhou Center for Inspection and Testing for Quality and Safety for Agricultural and Genetically Modified Products, Ministry of Agriculture, PR China (http://www.nybzjhz.com/index.asp) following standard methods for the quantification of nutrients and organic matter. Briefly, the moisture content of the swine manure and larvae casts was determined after drying at 105°C for 24 h. Organic matter (OM) was determined the weight difference before and after heating at 550°C for 4 h. Total Kjeldahl nitrogen (TKN) was determined as alkali NH3 liberation following nitrogen conversion to NH4+-N by digestion with concentrated H2SO4 and HClO4. Total phosphorus (TP) was analyzed using the molybdenum antimony colorimetric method after digestion in concentrated H2SO4 and HClO4. Total potassium (TK) was determined after digesting 0.3 g dry sample in a di-acid mixture (HNO3:HClO4 = 4:1, v/v) by flame photometry.
Biochemical measurements
Four enzymes related to the biodegradation of carbon (C), nitrogen (N) and phosphorus (P) were selected as indicators of microbial activities: β-1,4-glucosidase (βG), cellobiohydrolase (CBH), β-1,4-N-acetylglucosaminidase (NAG) and acid phosphatase (AP). Fluorometric enzyme assays for βG, CBH, NAG and AP were conducted for manure samples using methylumbelliferyl-linked (MUB) substrates as previously described28,46. Briefly, sample suspensions were prepared by homogenizing 1 g (wet weight) of sample with 125 mL of 50 mmol L−1 sodium acetate buffer (pH 6.5) using a vortex shaker for 1 min. Assays were conducted in 96-well blank fluorescent plates (Corning Inc., costar 3603, USA). The substrates for the four enzymes were 4-MUB-β-D-glucoside, 4-MUB-β-D-cellobioside, 4-MUB-N-acetyl-β-D-glucosaminide and 4-MUB-phosphate, for βG, CBH, NAG and AP, following a previously described protocol46. The micro-plates were covered and incubated in the dark at 20°C for 4 h. At the end of the assay, 10 μL of 1.0 mol L−1 NaOH were added to each well to stop the reaction and induce fluorescence. Fluorescence was measured 1 min after NaOH addition to reduce signal noise28. Fluorescence was measured with a Bio-Tek Synergy HT microplate reader (Bio-Tek Inc., Winooski, VT, USA) with 365 nm excitation and 460 nm emission filters. Enzyme activities were calculated and expressed as nmol h−1 g−1.
Microbial respiration rate was estimated as the rate of CO2 evolution according to a previously described method24. Samples (10 g wet weight equivalent) were incubated in airtight containers at 24°C in the dark. Evolved CO2 was captured with a 1.0 mol L−1 NaOH solution, which was titrated with HCl to the phenolphthalein endpoint.
454 pyrosequencing of bacterial communities in manure and vermicompost
Community DNA was extracted from 0.2 g of samples using the Ezup genomic DNA extraction kit (Sangon Biotech, Shanghai, China) according to the manufacturer's protocol. Extracted DNA was quantified using a Nanodrop spectrophotometer (ND-1000, NanoDrop Technologies, Wilmington). An aliquot (50 ng) of purified DNA from each sample were used as the template for amplification of the V3–V6 regions of bacterial 16S rRNAs using the following primer set47: 341F (5-CCTACGGGAGGCAGCAG-3) with the Roche 454 ‘A’ pyrosequencing adapter and a unique 7 bp barcode sequence and 1073R(5- ACGAGCTGACGACARCCATG-3) with the Roche 454 ‘B’ sequencing adapter at the 5-end of each primer respectively. Polymerase chain reaction (PCR) were carried out in 20 μL reaction mixtures containing 4 μL 5 × FastPfu Buffer, 2 μL 2.5 mmol L−1 dNTPs, 0.8 μL 5 μmol L−1 Forward Primer, 0.8 μL 5 μmol L−1 Reverse Primer, 0.4 μL FastPfu Polymerase, 10 ng Template DNA and add ddH2O to 20 μL. Each reaction mix was amplified under the following conditions: 2 minutes at 95°C, 25 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s and extension at 72°C for 30 s; with a final extension at 72°C for 5 min. PCR products were pooled and purified by Agarose Gel DNA purification kit (Sangon Biotech, Shanghai, China). The PCR products from each sample were combined in equimolar ratio in a single tube and run on a Roche FLX 454 sequencer (Roche Diagnostics Corporation, Branford, CT, USA). Sequences have been deposited into the NCBI short-reads archive database (Accession number: SRA00128). Procedures of sequence analysis were provided in the supplemental materials (S2).
Statistical analysis
All assays were conducted in triplicates. The cumulative reduction rate, daily reduction rate, cumulative reduction percentage and daily reduction percentage of the vermicompost antibiotics, OM, TKN, TP or TK were estimated by these formulas:




where CRi, Cvi are the antibiotic, OM, TKN, TP or TK concentrations of raw manure and vermicompost on the i-th day, respectively (Table S1, Fig. S1). MRi, MVi are manure inputs and mass of vermicompost left on the i-th day. S is the basal area of one vermireactor. Ti is vermicomposting time on the i-th day.
Analysis of variance (ANOVA) was performed with SPSS Statistical software version 17.0, using the S-N-K test for multiple comparison. The relationships among taxonomic diversity in the microbial communities and antibiotic attenuation and environmental variables were evaluated with redundancy analysis (RDA) using CANOCO 4.5, with phylum/class data entered as supplementary variables.
References
Wang, H., Zhang, Z. J., Czapar, G. F. & Zheng, J. G. A full-scale house fly (Diptera: Muscidae) larvae bioconversion system for value-added swine manure reduction. Waste Manage. Res. 31, 223–231 (2013).
Westerman, P. & Bicudo, J. Management considerations for organic waste use in agriculture. Bioresour. Technol. 96, 215–221 (2005).
Yehuda, B. et al. Bioconversion of poultry and fish waste by Lucilia Sericata and Sarcophaga Carnaria larvae. Asian J. Wat. Environ. Pollut. 8, 69–75 (2011).
Li, Q. et al. Bioconversion of dairy manure by black soldier fly (Diptera: Stratiomyidae) for biodiesel and sugar production. Waste Manage. 31, 1316–1320 (2011).
Zhang, Z., Wang, H., Zhu, J., Suneethi, S. & Zheng, J. Swine manure vermicomposting via housefly larvae (Musca domestica): The dynamics of biochemical and microbial features. Bioresour. Technol. 118, 563–571 (2012).
Zheng, L. Y. et al. Bacteria mediate oviposition by the black soldier fly, Hermetia illucens (L.), (Diptera: Stratiomyidae). Sci. Rep. 3, 2563 (2013)
Čičková, H. et al. Biodegradation of pig manure by the housefly, Musca domestica: A viable ecological strategy for pig manure management. PloS One 7, e32798 (2012).
Castillo, J. M., Romero, E. & Nogales, R. Dynamics of microbial communities related to biochemical parameters during vermicomposting and maturation of agroindustrial lignocellulose wastes. Bioresour. Technol. 146, 345–354 (2013).
Zhu, F. X. et al. Rapid production of maggots as feed supplement and organic fertilizer by the two-stage composting of pig manure. Bioresour. Technol. 116, 485–491 (2012).
Chee-Sanford, J. C. et al. Fate and transport of antibiotic residues and antibiotic resistance genes following land application of manure waste. J. Environ. Qual. 38, 1086–1108 (2009).
Huang, Y. J. et al. Simultaneous extraction of four classes of antibiotics in soil, manure and sewage sludge and analysis by liquid chromatography-tandem mass spectrometry with the isotope-labelled internal standard method. Anal. Methods 5, 3721–3731 (2013).
Wu, N., Qiao, M., Zhang, B., Cheng, W. D. & Zhu, Y. G. Abundance and diversity of tetracycline resistance genes in soils adjacent to representative swine feedlots in China. Environ. Sci. Technol. 44, 6933–6939 (2010).
Ho, Y. B., Zakaria, M. P., Latif, P. A. & Saari, N. Degradation of veterinary antibiotics and hormone during broiler manure composting. Bioresour. Technol. 131, 476–484 (2013).
Ramaswamy, J., Prasher, S. O., Patel, R. M., Hussain, S. A. & Barrington, S. F. The effect of composting on the degradation of a veterinary pharmaceutical. Bioresour. Technol. 101, 2294–2299 (2010).
Awad, Y. M. et al. Veterinary antibiotics contamination in water, sediment and soil near a swine manure composting facility. Environ. Earth Sci. 71, 1433–1440 (2014).
Kim, K.-R. et al. Decline in extractable antibiotics in manure-based composts during composting. Waste Manage. 32, 110–116 (2012).
Arikan, O., Mulbry, W., Ingram, D. & Millner, P. Minimally managed composting of beef manure at the pilot scale: effect of manure pile construction on pile temperature profiles and on the fate of oxytetracycline and chlortetracycline. Bioresour. Technol. 100, 4447–4453 (2009).
Selvam, A., Zhao, Z. & Wong, J. W. Composting of swine manure spiked with sulfadiazine, chlortetracycline and ciprofloxacin. Bioresour. Technol. 126, 412–417 (2012).
Dolliver, H., Gupta, S. & Noll, S. Antibiotic degradation during manure composting. J. Environ. Qual. 37, 1245–1253 (2008).
Li, B. & Zhang, T. Biodegradation and adsorption of antibiotics in the activated sludge process. Environ. Sci. Technol. 44, 3468–3473 (2010).
Dorival-Garcia, N., Zafra-Gomez, A., Navalon, A., Gonzalez, J. & Vilchez, J. L. Removal of quinolone antibiotics from wastewaters by sorption and biological degradation in laboratory-scale membrane bioreactors. Sci. Total Environ. 442, 317–328 (2013).
Huang, C.-H., Renew, J. E., Smeby, K. L., Pinkston, K. & Sedlak, D. L. Assessment of potential antibiotic contaminants in water and preliminary occurrence analysis. J. Contemp. Wat. Res. Edu. 120, 30–40 (2011).
Ali, M. et al. Effect of redox potential and pH status on degradation and adsorption behavior of tylosin in dairy lagoon sediment suspension. Chemosphere 91, 1583–1589 (2013).
Aira, M., Monroy, F. & Domínguez, J. Microbial biomass governs enzyme activity decay during aging of worm-worked substrates through vermicomposting. J. Environ. Qual. 36, 448–452 (2007).
Aira, M. & Domínguez, J. Earthworm effects without earthworms: inoculation of raw organic matter with worm-worked substrates alters microbial community functioning. PloS One 6, e16354 (2011).
Zhang, T. & Li, B. Occurrence, transformation and fate of antibiotics in municipal wastewater treatment plants. Critical Rev. Environ. Sci. Technol. 41, 951–998 (2011).
Zhang, H. C. & Huang, C. H. Oxidative transformation of fluoroquinolone antibacterial agents and structurally related amines by manganese oxide. Environ. Sci. Technol. 39, 4474–4483 (2005).
Sinsabaugh, R. L. & Follstad Shah, J. J. F. Ecoenzymatic stoichiometry and ecological theory. In: Annual Review of Ecology, Evolution and Systematics, Futuyma, D. J., Ed., 43, 313–343 (Annual Reviews, Palo Alto, 2012).
Morar, M. & Wright, G. D. The genomic enzymology of antibiotic resistance. Annu. Rev. Genet. 44, 25–51 (2010).
Hemaiswarya, S., Kruthiventi, A. K. & Doble, M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 15, 639–652 (2008).
Wright, G. D. Bacterial resistance to antibiotics: Enzymatic degradation and modification. Adv. Drug Delivery Rev. 57, 1451–1470 (2005).
Fernandez-Gomez, M. J., Nogales, R., Insam, H., Romero, E. & Goberna, M. Use of DGGE and COMPOCHIP for investigating bacterial communities of various vermicomposts produced from different wastes under dissimilar conditions. Sci. Total Environ. 414, 664–671 (2012).
Monroy, F., Aira, M. & Dominguez, J. Reduction of total coliform numbers during vermicomposting is caused by short-term direct effects of earthworms on microorganisms and depends on the dose of application of pig slurry. Sci. Total Environ. 407, 5411–5416 (2009).
Su, Z. et al. Comparison of bacterial diversity in wheat bran and in the gut of larvae and newly emerged adult of Musca domestica (Diptera: Muscidae) by use of ethidium monoazide reveals bacterial colonization. J. Economic Entomol. 103, 1832–1841 (2010).
Zhao, L. et al. Earthworm–microorganism interactions: A strategy to stabilize domestic wastewater sludge. Wat. Res. 44, 2572–2582 (2010).
Sharmin, F., Wakelin, S., Huygens, F. & Hargreaves, M. Firmicutes dominate the bacterial taxa within sugar-cane processing plants. Sci. Rep. 3, 3107 (2013).
Thomas, F., Hehemann, J.-H., Rebuffet, E., Czjzek, M. & Michel, G. Environmental and gut Bacteroidetes: the food connection. Front. Microbiol. 2, 93 (2011).
Näslund, J., Hedman, J. E. & Agestrand, C. Effects of the antibiotic ciprofloxacin on the bacterial community structure and degradation of pyrene in marine sediment. Aquat. Toxicol. 90, 223–227 (2008).
Maki, T. et al. Bacterial degradation of antibiotic residues in marine fish farm sediments of Uranouchi Bay and phylogenetic analysis of antibiotic-degrading bacteria using 16S rDNA sequences. Fisheries Sci. 72, 811–820 (2006).
Martinez, J. L. Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ. Pollut. 157, 2893–2902 (2009).
Manichanh, C. et al. Reshaping the gut microbiome with bacterial transplantation and antibiotic intake. Genome Res. 20, 1411–1419 (2010).
Shoaie, S. et al. Understanding the interactions between bacteria in the human gut through metabolic modeling. Sci. Rep. 3, 2532 (2013).
Clayton, T. A. et al. Pharmacometabonomic identification of a significant host-microbiome metabolic interaction affecting human drug metabolism. Proc. Nat. Acad. Sci. 106, 14728–14733 (2009).
Fierer, N. et al. Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proc. Nat. Acad. Sci. 109, 21390–21395 (2012).
Li, J. et al. Plasmid-Mediated quinolone resistance genes and antibiotic residues in wastewater and soil adjacent to swine feedlots: Potential transfer to agricultural lands. Environ. Health Perspect. 120, 1144–1149 (2012).
Saiya-Cork, K., Sinsabaugh, R. & Zak, D. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol. Biochem. 34, 1309–1315 (2002).
Wu, G. D. et al. Sampling and pyrosequencing methods for characterizing bacterial communities in the human gut using 16S sequence tags. BMC Microbiol. 10, 206 (2010).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (41373074), National Ministry of Science and Technology (2013GB23600658) and Zhejiang Science and Technology Innovation Program (2013C33001, 2011F20025).
Author information
Authors and Affiliations
Contributions
Z.J.Z. and X.H.X. conceived and designed the study. J.G.S., H.W., C.F.Z. and M.L. conducted field sampling and biochemical & molecular sequencing. L.H.W., F.P. and H.Y.L. performed antibiotics analysis and data processing. Q.H. and H.W. operated statistic analysis. All authors assisted with drafting the manuscript. Z.J.Z., J.G.S., H.W. and X.H.X. revised the manusctipt.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Zhang, Z., Shen, J., Wang, H. et al. Attenuation of veterinary antibiotics in full-scale vermicomposting of swine manure via the housefly larvae (Musca domestica). Sci Rep 4, 6844 (2014). https://doi.org/10.1038/srep06844
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep06844
This article is cited by
-
Valorization of Cow Manure: Unraveling Bacterial Community Changes Driven by Vermicomposting and Their Impact on Vermicompost Tea Production
Waste and Biomass Valorization (2023)
-
Characterization and variation of dissolved organic matter in composting: a critical review
Frontiers of Environmental Science & Engineering (2023)
-
Evaluation of bacterial diversity in a swine manure composting system contaminated with veterinary antibiotics (VAs)
Archives of Microbiology (2023)
-
Enterobacter hormaechei in the intestines of housefly larvae promotes host growth by inhibiting harmful intestinal bacteria
Parasites & Vectors (2021)
-
Potentials and Limitations of the Bioconversion of Animal Manure Using Fly Larvae
Waste and Biomass Valorization (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.