Abstract
Reversible control of charge transport and magnetic properties without degradation is a key for device applications of transition metal oxides. Chemical doping during the growth of transition metal oxides can result in large changes in physical properties, but in most of the cases irreversibility is an inevitable constraint. Here we report a reversible control of charge transport, metal-insulator crossover and magnetism in field-effect devices based on ionically gated archetypal oxide system - SrRuO3. In these thin-film devices, the metal-insulator crossover temperature and the onset of magnetoresistance can be continuously and reversibly tuned in the range 90–250 K and 70–100 K, respectively, by application of a small gate voltage. We infer that a reversible diffusion of oxygen ions in the oxide lattice dominates the response of these materials to the gate electric field. These findings provide critical insights into both the understanding of ionically gated oxides and the development of novel applications.
Similar content being viewed by others
Introduction
Strongly correlated transition metal oxides are known for their rich physics, including ferromagnetism (FM), metal-insulator transition (MIT), high-temperature superconductivity, colossal magnetoresistance and multiferroicity1,2,3. Achieving control over these phenomena is one of the most pursued goals in condensed matter physics and materials science4,5. Recently, ionic liquid (IL) gating of organic semiconductors and inorganic oxides has been used to induce high densities of charge carriers (over 1014 cm−2) in these materials by an external electric field4,6,7,8,9,10,11. However, the mechanism of IL gating in transition metal oxides is still unclear, with the electrostatic and electrochemical doping mechanisms debated7,8,9,10,11,12,13. For instance, there are conflicting interpretations of IL gating effect on the metal-insulator transition in VO2. Nakano et al. interpreted gating induced modulations of metal-insulator transition as originated from the electrostatically induced surface charge accumulation that mediates bulk structural phase transformation over a macroscopic length scale10. On the contrary, Jeong et al. argued that IL gating leads to electrochemical doping, in which oxygen vacancies are created or annihilated in VO211. Indeed, when a gate voltage of a few volts is applied to the ionic liquid, electric field generated at the IL-VO2 interface can be sufficiently high to force oxygen ions to drift in or out of the oxide. Depending on the physical properties of a particular oxide and the experimental conditions, either of these mechanisms could be relevant, or a combination of two may govern the field-induced phenomena in ionically gated oxides.
SrRuO3 (SRO) is the most popular strongly correlated conducting oxide, with a FM transition temperature (TFM) of ~160 K, that can be seamlessly integrated with other oxides in oxide heterostructure electronics14,15. In addition, SRO is well known among oxides for the very high stability of its oxidative state (see [14-16] and refs. therein). Modifications of magnetic and transport properties of (ungated) SRO thin films were demonstrated by varying film thickness, partial pressure of O2 during the film growth and chemical doping15,16,17,18. For instance, lower TFM were observed in SRO films grown under low oxygen partial pressure, which can be explained by the presence of oxygen vacancies16. When oxygen vacancies are created, the average SRO unit cell volume increases, because the structure is relaxed by losing the bonding. Consequently, the orbital overlap between the ruthenium d-orbitals and oxygen p-orbitals is reduced, leading to a smaller density of states at the Fermi level and a lower ferromagnetic transition temperature relatively to TFM of the stoichiometric SRO. Metal-insulator crossover in (ungated) SRO has also been reported, with a metallic state (dρ/dT > 0) gradually transforming into an insulating one (dρ/dT < 0). The metal-insulator crossover temperature, TMI, increases, when the film thickness is reduced or the level of chemical impurities increases15,18. It is believed that TMI in this system is strongly affected by disorder. For instance, in SrTixRu1-xO3, increasing x induces a weak localization that eventually drives the system into an insulating state with a Mott-type variable range hopping transport - a behavior governed by a combination of disorder and electron correlations18.
Here we investigate the effect of electric field gating on charge transport and magnetic properties of SRO films by using ionically gated devices that utilize the recently developed facile “cut and stick” semi-solid ion gels (IG)19. This new method has substantially improved the mechanical integrity of ionically gated devices and allowed us for the first time to observe modulations of the metal-insulator crossover and ferromagnetism in SRO with a gate voltage.
Results
Ionically gated 10-monolayer (4 nm-thick) SRO films
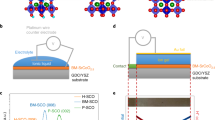
The configuration of our ionically gated SRO devices is shown in Fig. 1 (for fabrication details see Methods). In brief, a few monolayer (ML)-thick SRO thin films were grown on single-crystal SrTiO3 substrates by a pulsed laser deposition (PLD). Atomic force microscopy (AFM) reveals that the surface of these few nm-thick SRO films is smooth, with atomic terraces of the underlying strontium titanate substrate clearly visible. Source and drain contacts were deposited on SRO by sputtering Au through a shadow mask and a 10 μm-thick film of ion gel was attached on top of the sample, covering the area between the contacts (the channel). Flexible Au gate was then attached on top of the ion gel. Figure 1 also shows SRO crystal structure and the chemical structure of the ion gel. The cations and anions of the gel ([EMI]+ and [TFSA]−, respectively) are caged in a neutral polymer matrix, resulting in a semi-solid, easy-to-handle gel that makes device fabrication convenient and provides an effective capacitance of 4 μF·cm−2, comparable to that of the best ILs19.
Ion-gel gated SrRuO3 (SRO) thin film transistors.
(a) SrRuO3 has an orthorhombic structure with Ru inside the octahedral cage of six oxygen atoms. (b) Optical microphotograph of an ion-gel gated SRO device (before gate lamination) with an inset showing AFM image of a 4 nm-thick (10-monolayer) SRO grown at TiO2-terminated single-crystal SrTiO3 substrate. The topography clearly shows a smooth film that continuously coats the substrate terraces without a detrimental 3D growth. (c) Schematics of the ion-gel gated SRO transistor. S, D and G denote the source, drain and gate electrodes, respectively. The gate is formed by laminating a metalized 2.5 μm-thick Mylar film on top of the ion gel. (d) Chemical structure of the ion gel components used in this study.
To investigate the electric field effect in SRO, we have first measured the source-drain current, ISD, while sweeping the gate voltage, VG, in close loops between −2 and 2 V starting at VG = 0 and at a VG-sweep rate of 0.25 mV·s−1 (5 mV steps in 20 s intervals). Room temperature ISD(VG) characteristics exhibit a pronounced hysteresis, as shown in Fig. 2a. The sample's initial sheet resistivity per square, ρ, (that is, ρ before gating) is 800 Ω. With increasing gate voltage, ISD increases superlinearly with a threshold-like onset (sec. 1 of Supplementary Information)12, resulting in a more conducting state that persists even when VG is reduced back to 0. A further decrease of VG to −2 V results in the original more resistive state of the sample with ρ ≈ 800 Ω. Additional tests reveal that resistivity modulations with VG are persistent, as long as the devices are kept in high vacuum, but quickly decay if the ion gel is removed and SRO is exposed to air (sec. 2 and 3 of Supplementary Information). This behavior is consistent with redox reactions at the ion-gel/SRO interface.
Charge transport and magnetic properties of ionically gated 10-monolayer SrRuO3.
(a) Gate voltage dependence of the source-drain current (top) and gate leakage current (bottom) at room temperature (VG sweep rate is 0.25 mV·s−1). (b) Resistivity as a function of temperature measured at VG = 0, 2.2 and −2.2 V. TMI (arrows) shifts to lower temperatures with positive VG applied to the ion gel. The inset shows TMI as a function of VG obtained in the following gating sequence: VG = 0, 2, −2, 0, 1.5, −2.2 and 2.2 V. (c) Magnetic field dependence of magnetoresistance (Δρ/ρ) at various temperatures and VG = 0, VSD = 0.1 V. Magnetic field of up to ± 0.5 T was applied perpendicular to the film surface. (d) Temperature dependence of magnetoresistance, Δρ/ρ(T), obtained in 0.5 T at various VG. The systematic shift of the magnetoresistance onset toward lower temperatures is observed for VG > 0, while the changes at VG < 0 are negligible. The inset shows a semi-log plot of the same data. The magnetoresistance onset temperature, TC, is defined as the temperature, at which -Δρ/ρ drops below 0.01% (red arrows).
The ionic gate “leakage” current (that is, an electrolytic displacement current), IG, recorded during these measurements was about 10−8 A, which is negligible compared to the induced modulations in ISD in the entire VG sweep range (δISD/IG > 103). This clearly shows that the source-drain conductance modulation in our devices is not an artifact of the gate leakage. It is worth noting that water related impurities in ionic liquids may lead to artifacts in measurements of IL/oxide devices, as pointed out recently20,21. To prevent such effects, all the measurements in this work have been carried out in a “dry” state of ion-gel/SRO devices in high vacuum (sec. 4 of Supplementary Information, Methods). In addition, control experiments with ion-gel gated devices based on other (non-oxide) materials (single crystals of rubrene and WSe2) have been carried out (sec. 5 and 9 of Supplementary Information). These control tests indicate that the effects observed in our ion-gel/SRO are not related to water contamination. The main contribution to the displacement current, IG, comes from the motion of the molecular ions ([EMI]+ and [TFSA]−) of the ion gel in response to an applied VG (the so-called polarization of ionic liquids)19. This response is very fast: the capacitance (~4 μF·cm−2) of the ion gel remains almost frequency independent up to 100 kHz19. The reason the displacement process contributing to IG in the lower panel of Fig. 2a does not seem to saturate is that VG in this measurement is constantly changing (in a slow sweep), thus causing the ion gel polarization to follow VG with a similar rate.
Figure 2b shows the temperature dependence of resistivity measured at several fixed values of VG = 0, 2.2 and −2.2 V, that is, at the states marked by the three colored symbols on the room-temperature hysteresis loop (Fig. 2a). VG was always applied at room temperature before measuring ρ(T). The normalized sheet resistivity, ρ/ρ260K, plotted as a function of T clearly shows a shift of TMI with VG. TMI is defined as the temperature, at which resistivity reaches the minimum, dρ/dT = 0 (see also Supplementary Fig. S6). At VG > 0, SRO film becomes more conducting with a lower TMI (red curve), compared to the state at zero gate voltage. At VG = − 2.2 V, ρ(T) dependence of the sample resembles that at VG = 0 (green and black curves), consistent with the hysteresis in ISD(VG) measurements in Fig. 2a. The inset in Fig. 2b shows TMI as a function of VG obtained from a series of ρ(T) measurements at different VG values. It is clear that the metal-insulator crossover temperature can be tuned in a wide range of 50 < TMI < 120 K by application of a small gate voltage, −2.2 < VG < 2.2 V. In addition, modulations of ρ with VG are quantitatively and qualitatively different on the insulating and metallic sides of the crossover (sec. 6 of Supplementary Information). We have systematically observed the effect of gating on the metal-insulator crossover in our 6 ML and 10 ML SRO samples. It's worth noting that we have not detected any kink in ρ(T) in our as-grown SRO samples before applying ion gel (sec. 7 of Supplementary Information). A kink in ρ(T) is believed to be associated with a ferromagnetic transition in the magnetic structure of SRO15,17. Nevertheless, our magnetization measurements, M(T), clearly show a ferromagnetic transition in our SRO films with the FM onset temperature, TFM ~ 100 K (sec. 8 of Supplementary Information). We believe the insulating state observed in ρ(T) below TMI and the absence of the kink in the resistivity measurements in our samples are related to disorder.
Figure 2c shows the magnetoresistance (MR) that we define as Δρ/ρ ≡ (ρB≠0 - ρB = 0)/ρB = 0. It is measured in a 10 ML SRO film at various temperatures and fixed VG = 0 (ungated device) and VSD = 0.1 V. The MR is negative and at 25 K it clearly shows a hysteresis with minima at the field of magnetic coercivity, HC ~ 1 kOe (black curve). On warming, the hysteresis and MR both reduce. Figure 2d shows the MR measured at B = 0.5 T as a function of temperature at the following sequence of gate voltages: VG = 0, 2, −2, 0, 1.5, −2.2 and 2.2 V. With positive gate voltage, the MR curves systematically shift toward lower temperatures (magenta, brown and red symbols). However, when a negative VG is applied (green stars), the system returns to the initial state corresponding to VG = 0 (blue diamonds and black squares). Note that since the field effect in this system depends on VG and the gating history, the absolute value of the compensating gate voltage or stressing time have to be adjusted in order to bring the system back to the initial state. The inset is a semi-log plot of the same MR(T) data. Here, we can define the so-called onset of magnetoresistance, characterized by the temperature, TC, at which Δρ/ρ drops below 0.01%, as indicated in the inset by the red arrows. Below this value, MR is very difficult to determine accurately. TC defined in such a way is consistent with the ferromagnetic transition temperature, TFM, obtained from the magnetization measurements (sec. 8 of Supplementary Information). Note that it has been reported that TFM in (ungated) SRO can be modified by chemical doping at Ru site with various transition metals and the onset temperature of MR consistently followed the change in TFM17. This suggests that electrical measurements (that is, magnetoresistance and its onset at TC) can be used to (indirectly) identify the ferromagnetic phase transition and its temperature TFM. The onset of magnetoresistance in our SRO devices can be systematically shifted between about 70 and 100 K by applying a small gate voltage of |VG| ≤ 2.2 V to the ion gel.
Ionically gated 6-monolayer SRO films
It has been previously observed that resistivity and the metal-insulator crossover in (ungated) SRO thin films strongly depend on the film thickness: with a decreasing number of layers, ρ and TMI both increase15. Furthermore, it has been shown that when the film thickness is reduced to 4-5 MLs, the sample's resistivity can increase by more than 8 orders of magnitude, compared to thicker samples and it becomes insulating in the entire accessible temperature range, T < 300 K15. In order to verify that similar thickness-dependent effects take place in our gated SRO devices, we have performed transport measurements in a device based on a thinner (6 ML) film (Fig. 3). ρ(T, VG) of the 6 ML SRO device shows that the film is about 3 times more resistive than the 10 ML device (zero-VG sheet resistivity is 2.4 kΩ at 270 Κ) and its TMI (~210 K) is twice as high as that of the 10 ML sample. The shift of TMI to a higher temperature is related to a greater disorder typical for thinner films. Indeed, in very thin films, disorder related to defects, twin domains and FM domain boundaries is more critical, leading to a stronger charge carrier localization. ρ(T) of the 6 ML device was measured at different gate voltages following the sequence: VG = 0 (black), 2.5 (red), −2.5 (green) and 3 V (magenta). The slope of ρ(T) indicates that the behavior of the device at VG = 3 V is metallic in a wide temperature range T > 90 K (magenta). However, at VG = −2.5 V, the behavior is insulating in the entire temperature range (green). At the lowest temperature in our measurements (here, 30 K), the sample's resistivity is modulated with VG by more than a factor of 3. The inset shows the normalized resistivity vs. T: the arrows indicate TMI at various gate voltages. It is obvious that TMI is strongly affected by gating - it changes by more than 150 K with VG varied from −2.5 to 3 V. The drastic changes in ρ(T) dependence, including the absolute value of ρ and the metal-insulator crossover temperature shown in Fig. 3, clearly indicate that the ion-gel gating allows us to control the metal-insulator crossover in SRO. Note that the modulation of these properties with VG is noticeably stronger in the thinner (more insulating) sample.
Temperature dependence of resistivity of 6-monolayer SrRuO3 at different gate voltages.
ρ(T) was measured in the following order: VG = 0 (black), 2.5 (red), −2.5 (green) and 3 V (magenta). The inset shows the normalized resistivity vs. temperature (the arrows indicate the metal-insulator crossover temperature at different VG). TMI is reversibly modulated in the range 90–250 K by applying a small gate voltage, |VG| ≤ 3 V.
VG sweep rate dependence of the field effect in ionically gated SRO devices
In this work, we have demonstrated a control of the resistivity, the metal-insulator crossover and the onset of magnetoresistance in thin films of a strongly correlated oxide, SrRuO3, achieved in ionically gated field-effect devices. Magnetism in SRO has been interpreted as a Stoner-type itinerant ferromagnetism with the ferromagnetic transition temperature strongly affected by the density of states at the Fermi level and electron-electron interactions14. Thus, magnetism in SRO can be influenced by adding or removing charge carriers via an electric field effect. In addition, removing oxygen from SRO (that is, generating oxygen vacancies) can reduce the hybridization strength between d-orbitals of ruthenium and p-orbitals of oxygen, leading to a reduced density of states at the Fermi level. Therefore, modulations of TC and TMI observed in this study can, in principle, be explained by either an electrostatic carrier accumulation or an electrochemical doping (generation of oxygen vacancies). Indeed, when a positive gate voltage is applied to the ion gel, electrons should be electrostatically accumulated in the interfacial layer(s) of SRO, leading to an enhancement of conductivity. Removal of oxygen from transition metal oxides is typically equivalent to an electron doping and an enhancement of conductivity can also be expected in oxygen deficient samples. Note that an increased conductivity was observed in (ungated) oxygen deficient SRO16. Other physical properties of transition metal oxides can also drastically change with oxidative state of the system22.
In order to understand which of the above two mechanisms plays the dominant role in the reversible tuning of the metal-insulator crossover and magnetoresistance in our ionically gated SRO, we have examined the VG-sweep rate dependence of the field effect in our devices (Fig. 4a). Note that the systems dominated by fast electronic carrier accumulation, for instance ZnO FETs, show largely VG-sweep-rate independent characteristics23. Such dependence has been found to play a major role in organic field-effect transistors (OFET) based on certain disordered organic semiconductors24. In OFETs, it has been found that the nominal mobility calculated from the slope of transfer characteristics can vary by more than an order of magnitude, depending on how fast VG is swept24. This effect was ascribed to a gradual relaxation of the injected charge carriers in energetically wide distribution of trap states in disordered organic films and it highlights the necessity of more careful data interpretation in OFETs. In our ionically gated SRO, VG has been swept extremely slowly: in 5 mV increments with a time constant τ = 5–30 s per increment. Figure 4a shows a normalized resistivity, ρ(VG)/ρ(VG = 1.9V), monitored as VG is swept at different sweep rates, dVG/dt = 0.17, 0.25, 0.5 and 1 mV·s−1 (not in this order), achieved by varying τ while keeping the voltage increment fixed at 5 mV. The inset shows the resultant dependence of the slope of resistivity, dρ/dVG, on the VG-sweep rate. The choice of the sweep rates is based on typical diffusion time (10–100 min) of oxygen through a layer with thickness equal to the screening length in SRO (~5 Å). It is clear that when the sample is given more time to respond to the changing gate voltage, a greater change in ρ occurs. The most striking observation, however, is an extremely slow dynamics of the sample's response: indeed, the time constants in ρ(VG) measurement in the main panel of Fig. 4a are τ = 5, 10, 20 and 30 s (per 5 mV increment in VG). Even at the lowest VG-sweep rate of 0.17 mV·s−1, corresponding to the longest time constant of 30 s, the effect is far from being saturated: |dρ/dVG| is the highest. This dynamics is much slower than that observed in OFETs24,25. Such a remarkably slow process in an inorganic material cannot be rationalized in terms of electronic carrier relaxation in trap states in the FET's accumulation channel. Indeed, charge carrier mobilities in inorganic materials are typically much greater than those in van der Waals organic semiconductors and thus electronic carrier relaxation is expected to occur much faster here than in OFETs. Ionic motion in the ion gel can not account for such a slow dynamics either: measurements of the capacitance of the ion gel used in this work have shown that its capacitance (~4 μF·cm−2) remains almost frequency independent up to 100 kHz19.
ρ(VG) measurements in an ion-gel gated SRO transistor at different VG sweep rates.
(a) ρ(VG) recorded at room temperature as VG is increased at different rates, 0.17, 0.25, 0.5 and 1 mV·s−1 (indicated), corresponding to the time constants of 30, 20, 10 and 5 s between the 5 mV increments in VG, respectively. The inset shows the resultant dependence of the slope, dρ/dVG, on the gate voltage sweep rate. (b) Illustration of the mechanism of the electric field effect in ionically gated oxides. When VG > 0 is applied and the ion gel is polarized, negative [TFSA]− ions accumulate near the gate electrode, while positive [EMI]+ ions accumulate near the SrRuO3/ion-gel interface. A strong electric field generated in the top layers of the oxide drives oxygen ions out of the oxide lattice, which is equivalent of generating oxygen vacancies (shown as “v”). These vacancies are responsible for the observed changes in the transport and magnetic properties of ionically gated SRO.
VG sweep rate dependence in layered inorganic (non-oxide) semiconductor, WSe2
To exclude any remaining possibility that some extremely slow ionic motion in the ion gel might have caused the observed dynamics in our SRO devices, we have performed a control experiment, in which we used a different (non-oxide) material: layered transition metal dichalcogenide, WSe2. Conventional (non-IL) high-mobility field effect transistors based on these layered inorganic semiconductors were demonstrated as early as 200426. The ionically gated devices were prepared on WSe2 following the same protocol and using the same ion gel as on SRO and the field effect, ρ(VG), was measured at different VG-sweep rates at otherwise similar conditions (sec. 9 of Supplementary Information). Remarkably, almost no VG-sweep rate dependence could be detected in these devices (Fig. S9). This confirms that the slow dynamics observed in the SRO transistors is indeed related to the oxygen motion in the oxide. It's important that the strong VG-sweep rate dependence in SRO FETs is observed both at positive and negative VG (sec. 9 of Supplementary Information). This indicates that accumulation of either [TFSA]− anions or [EMI]+ cations results in a slow, rate dependent, reversible oxygen drift in the oxide.
Discussion
Several further control experiments lend an additional support to the model of electrochemical oxygen vacancy creation/annihilation. We have measured ρ(T) with VG applied at 220 K and compared it with the case of VG applied at room temperature (sec. 10 of Supplementary Information): this experiment clearly shows that there is almost no gating effect and no TMI shift, when VG is applied at low temperature. In a separate experiment, it was shown that the strength and hysteresis of the field effect in ISD(VG) characteristics measured at low temperatures are drastically reduced (sec. 11 of Supplementary Information). In addition, performing measurements of ion-gel/SRO devices in oxygen atmosphere results in a much stronger suppression of conductivity (depletion) at a negative VG (sec. 12 of Supplementary Information), consistent with the observation in other oxides11,27. Finally, we have estimated the carrier density expected in purely electrostatic charge accumulation model (following the procedure described in [20]) and that in the electrochemical doping scenario (sec. 13 of Supplementary Information). These calculations show that the electrochemical model describes the operation of ion-gel/SRO devices much better. It is worth noting that although a combination of electrostatic and electrochemical processes has been suspected to govern the field effect in certain IL-gated oxides, disentangling the relative contributions of these effects simply based on ISD(VG) loops measured at room temperature has been difficult20,21,28.
The combination of experiments performed in this work leads to a set of observations that are consistent with each other and strongly suggest that the dominant mechanism of the field effect in ionically gated SRO devices is an electric field induced migration of ionic oxygen in SRO lattice (creation and annihilation of oxygen vacancies). A positive gate voltage applied to the ion gel attracts the organic anions to the gate electrode, while the organic cations are accumulated near the interface with SRO (Fig. 4b). The electric field created by the accumulated cations drives the oxygen ions from SRO toward the ion gel, thus creating oxygen vacancies in the oxide. This effect is reversible on changing the gate voltage polarity.
To conclude, we have achieved a gate controlled electrochemical modulation of the metal-insulator crossover and the onset of magnetoresistance (associated with a ferromagnetism) in ion-gel gated thin-film SRO. The temperature of the metal-insulator crossover can be continuously and reversibly tuned in the range 90–250 K (in 6-ML SRO) and 50–120 K (in 10-ML SRO) by applying a small gate voltage, |VG| ≤ 3 V. The magnetoresistance onset can also be reversibly shifted by as much as 30 K. We have observed that modulations of ρ with VG depend strongly on the gate voltage sweep rate, with remarkably long time constants of tens of seconds, which can only be consistent with a slow dynamics of ionic species (such as oxygen) in the oxide lattice. We believe that the observed field effect on the electronic properties of SRO is predominantly governed by the creation and annihilation of oxygen vacancies, rather than an electrostatic accumulation of electrons or holes in SRO. Given the outstanding oxygen stability of SRO, demonstration of these effects in this material indicates that oxygen drift in the lattice might be even stronger in other ionically gated oxides. These fascinating switchable effects in magnetism and the metal-insulator crossover in ionically gated SRO help to improve our fundamental understanding of strongly correlated oxides and may point to novel electronic applications based on these materials.
Methods
SRO thin film growth and device fabrication
6 and 10 ML (a few nm-thick) SRO thin films were grown on TiO2-terminated SrTiO3 substrates using a pulsed laser deposition. For 10 ML SRO films, a pulsed KrF excimer laser with a power of 2 mJ·cm−2, a repetition rate of 1 Hz and a pulse duration of 10 ns has been used to ablate a target (99.9% SrRuO3, MTI Corp.) for 100 s in 50 mTorr of oxygen. The x-ray scattering data for 10 and 70 ML SRO films suggest that our as-deposited samples are slightly oxygen deficient (sec. 14 of Supplementary Information). For the 6 ML samples, the same power and frequency were used for 86 sec in 80 mTorr of oxygen. The film thickness was monitored in-situ using a reflection high-energy electron diffraction (RHEED). 60 nm-thick Au source and drain contacts were deposited on top of the SRO film using a dc magnetron sputtering through a shadow mask. “Cut and stick” rubbery ion gel layer was placed on top of the SRO sample, overlapping with the contacts. For the gate electrode, a 60 nm-thick gold film was sputtered at the surface of a free-standing 2.5 μm-thick flexible Mylar sheet. The metallized Mylar was then laminated on top of the ion gel (Au facing the gel), thus completing the device fabrication (Fig. 1). The chemical structure of the ion gel used in this study is shown in Fig. 1d. It consists of 1-ethyl-3-methylimidazolium, [EMI]+ and bis(trifluoromethylsulfonyl), [TFSA]−, - cations and anions, respectively, that are caged in a matrix of poly(vinylidene fluoride-co-hexa-fluoropropylene), P(VDF-HFP)19. Compared to ionic liquids, ion gels are semi-solid and do not flow, which makes device fabrication convenient. It can be used in any configuration, because ions are trapped in the polymer matrix. Furthermore, ion gels can be easily cut in any shape with a razor blade and applied to any surface of interest, providing an effective capacitance of ~4 μF·cm−2, similar to that of the best ILs.
Electrical measurements
In order to minimize incorporation of water, measurements have been carried out in “dry” conditions: (a) all the components of our devices (SRO films and ion gel) were stored in a dry box, (b) they were exposed to air only for a few minutes during lamination of ion gel on SRO, (c) the assembled devices were quickly loaded into a high-vacuum chamber and pumped with a dry pump to 10−6 Torr, where they were kept for at least 3 h before measurements.
Electrical measurements were performed using Keithley source-meters K-2400 and electrometer K-6514 controlled by a LabView program. Advanced Research Systems cryostat and GMW 5403 electromagnet were used for temperature variable magneto-transport measurements. In T-variable measurements, VG was always changed at room temperature, before the temperature cycling. In ρ(T) measurements, temperature was slowly lowered below 300 K at a controlled rate of 4 K/min in the cryostat while recording ρ(T). Measurements were performed in high vacuum (10−6 Torr). The dc magnetization was measured using a superconducting quantum interference device magnetometer (Quantum Design magnetic property measurement system).
References
Imada, M., Fujimori, A. & Tokura, Y. Metal-insulator transitions. Rev. Mod. Phys. 70, 1039–1263 (1998).
Jin, S. et al. Thousandfold Change in Resistivity in Magnetoresistive La-Ca-Mn-O Films. Science 264, 413–415 (1994).
Cheong, S. W. & Mostovoy, M. Multiferroics: a magnetic twist for ferroelectricity. Nat. Mater. 6, 13–20 (2007).
Ueno, K. et al. Field-Induced Superconductivity in Electric Double Layer Transistors. J. Phys. Soc. Jpn. 83, 032001 (2014).
Ahn, C. H. et al. Electrostatic modification of novel materials. Rev. Mod. Phys. 78, 1185–1212 (2006).
Kim, S. H. et al. Electrolyte-Gated Transistors for Organic and Printed Electronics. Adv. Mater. 25, 1822–1846 (2013).
Dhoot, A. S., Israel, C., Moya, X., Mathur, N. D. & Friend, R. H. Large Electric Field Effect in Electrolyte-Gated Manganites. Phys. Rev. Lett. 102, 136402 (2009).
Bollinger, A. T. et al. Superconductor-insulator transition in La2-xSrxCuO4 at the pair quantum resistance. Nature 472, 458–460 (2011).
Shi, J., Ha, S. D., Zhou, Y., Schoofs, F. & Ramanathan, S. A. correlated nickelate synaptic transistor. Nat. Commun. 4, 2676 (2013).
Nakano, M. et al. Collective bulk carrier delocalization driven by electrostatic surface charge accumulation. Nature 487, 459–462 (2012).
Jeong, J. et al. Suppression of Metal-Insulator Transition in VO2 by Electric Field-Induced Oxygen Vacancy Formation. Science 339, 1402–1405 (2013).
Zhou, Y. & Ramanathan, S. Relaxation dynamics of ionic liquid-VO2 interfaces and influence in electric double-layer transistors. J. App. Phys. 111, 084508 (2012).
Fujiwara, K., Ichimura, T. & Tanaka, H. Nonvolatile Transport States in Ferrite Thin Films Induced by Field-Effect Involving Redox Processes. Adv. Mat. Interfaces (Admi.201300108).
Allen, P. B. et al. Transport properties, thermodynamic properties and electronic structure of SrRuO3 . Phys. Rev. B 53, 4393–4398 (1996).
Xia, J., Siemons, W., Koster, G., Beasley, M. R. & Kapitulnik, A. Critical thickness for itinerant ferromagnetism in ultrathin films of SrRuO3 . Phys. Rev. B 79, 140407R (2009).
Lu, W. et al. Effect of oxygen vacancies on the electronic structure and transport properties of SrRuO3 thin films. J. Appl. Phys. 113, 17E125 (2013).
Pi, L., Maignan, A., Retoux, R. & Raveau, B. Substitution at the Ru site in the itinerant ferromagnet SrRuO3 . J. Phys.: Condens. Matter 14, 7391–7398 (2002).
Kim, K. W., Lee, J. S., Noh, T. W., Lee, S. R. & Char, K. Metal-insulator transition in a disordered and correlated SrTi1-xRuxO3 system: Changes in transport properties, optical spectra and electronic structure. Phys. Rev. B 71, 125104 (2005).
Lee, K. H. et al. “Cut and Stick” Rubbery Ion Gels as High Capacitance Gate Dielectrics. Adv. Mater. 24, 4457–4462 (2012).
Ha, S. D., Vetter, U., Shi, J. & Ramanathan, S. Electrostatic gating of metallic and insulating phases in SmNiO3 ultrathin films. Appl. Phys. Lett. 102, 183102 (2013).
Ji, H., Wei, J. & Natelson, D. Modulation of the Electrical Properties of VO2 Nanobeams Using an Ionic Liquid as a Gating Medium. Nano Lett. 12, 2988–2992 (2012).
Jeen, H. et al. Reversible redox reactions in an epitaxially stabilized SrCoOx oxygen sponge. Nature Mater. 12, 1057–1063 (2013).
Maeng, J. et al. Effect of gate bias sweep rate on the electronic properties of ZnO nanowire field-effect transistors under different environments. Appl. Phys. Lett. 92, 233120 (2008).
Chen, Y. et al. Dynamic character of charge transport parameters in disordered organic semiconductor field-effect transistors. Phys. Chem. Chem. Phys. 14, 14142–14151 (2012).
Chen, Y. & Podzorov, V. Bias Stress Effect in “Air-Gap” Organic Field-Effect Transistors. Adv. Mater. 24, 2679–2684 (2012).
Podzorov, V., Gershenson, M. E., Kloc, Ch., Zeis, R. & Bucher, E. High-mobility field-effect transistors based on transition metal dichalcogenides. Appl. Phys. Lett. 84, 3301 (2004).
Schladt, T. D. et al. Crystal-Facet-Dependent Metallization in Electrolyte-Gated Rutile TiO2 Single Crystals. ACS Nano 7, 8074 (2013).
Ichimura, T., Fujiwara, K. & Tanaka, H. Dual field effects in electrolyte-gated spinel ferrite: electrostatic carrier doping and redox reactions. Sci. Rep. 4, 5818 (2014).
Acknowledgements
HTY and the part of work performed at Rutgers University have been financially supported by DOE BES award under Grant No. DE-SC0005464 (ER46763). SWC and BG are funded by the Gordon and Betty Moore Foundation's EPiQS Initiative through Grant GBMF4413 to the Rutgers Center for Emergent Materials. We are grateful to C. D. Frisbie and L. C. Feldman for helpful discussions.
Author information
Authors and Affiliations
Contributions
H.T.Y. and V.P. designed the experiments. H.T.Y. performed device fabrication and measurements. B.G. and S.W.C. grew SrRuO3 films. W.X. prepared the ion gel. H.T.Y. and V.P. analyzed the data and wrote the paper. All authors discussed the results and contributed to editing the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Information: Tuning the metal-insulator crossover and magnetism in SrRuO3 by ionic gating.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Yi, H., Gao, B., Xie, W. et al. Tuning the metal-insulator crossover and magnetism in SrRuO3 by ionic gating. Sci Rep 4, 6604 (2014). https://doi.org/10.1038/srep06604
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep06604
This article is cited by
-
Reversible metal-insulator transition in SrIrO3 ultrathin layers by field effect control of inversion symmetry breaking
Communications Materials (2023)
-
Emergent electric field control of phase transformation in oxide superlattices
Nature Communications (2020)
-
Reversible manipulation of the magnetic state in SrRuO3 through electric-field controlled proton evolution
Nature Communications (2020)
-
Electrolyte-based ionic control of functional oxides
Nature Materials (2019)
-
Reversible, Electric-Field Induced Magneto-Ionic Control of Magnetism in Mesoporous Cobalt Ferrite Thin Films
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.