Abstract
Variation in TP53 has been associated with cancer. The pro-allele of a TP53 polymorphism in codon 72 (rs1042522) has been associated with longevity. Recently, we showed that the same allele might be involved in preservation of glucose metabolism, body composition and blood pressure during ageing. Here, we assessed glucose tolerance and body composition in mice carrying the human polymorphism. Our data do not support the previous findings in humans, suggesting that this polymorphism does not play a major role in development of glucose metabolism and body composition during ageing. Alternatively, the mouse model may not be suitable to validate these rs1042522-associated traits up to the age tested.
Similar content being viewed by others
Introduction
The human tumor protein p53, encoded by TP53, plays a major role in cell cycle arrest and apoptosis as result of DNA damage. Many mutations and polymorphisms in TP53 have been associated with cancer1,2. A common and well-described polymorphism is rs1042522 at codon 72, which is an arginine/proline variant. The pro-allele has been associated with cancer but, also with longevity3,4,5. It has been shown that the carriers of the pro-allele have less efficient apoptosis6,7. This might result in increased cancer susceptibility, but accompanied by preservation of stem cell stock. Hence, the pro-allele can result in improved longevity if cancer is prevented or survived. Recently, we found that pro/pro carriers are protected against increases in serum glucose levels, waist circumference and diastolic blood pressure during ageing, which might be a second, not mutually exclusive, explanation of the increased longevity associated with the same genotype8. However, the observed effect sizes are small and tested in only two cohorts. The human rs1042522 polymorphism has been constructed in two mouse models. These comprise a transgenic model in which both human genotypes were constructed in a p53−/− mouse and a knock-in model in which the endogenous mouse exon 4 was replaced by the human exon 4 carrying either the pro- or arg-allele7. In both models the apoptotic preference of the pro-allele was confirmed7. However, none of the associated metabolic traits observed in humans have been tested in these models. Here, we tested whether ageing-dependent differences in changes in glucose levels and body composition between pro/pro and arg/arg carriers could be detected in the knock-in mouse model.
Results
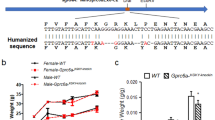
Mice homozygous for the pro-allele (P/P) and homozygous for the arg-allele (R/R) were generated in a pure C57BL6/J background using heterozygous breeding pairs (P/R). Wild type mice (normal mouse Trp53) were used as reference and were from our own breeding facility. All cohorts existed of 20 male mice and were maintained on standard chow diet. Body weights and food intake were monitored weekly and were identical for all genotypes (figure 1).
In order to assess glucose homeostasis oral glucose tolerance tests (ogtt) were performed at 10, 20 and 40 weeks of age. Glucose levels were assessed at 0, 15, 30, 60 and 120 minutes. Ageing-related changes of glucose measurements at the independent time points were compared using an age-by-genotype interaction in generalized estimating equations (figure 2). Ageing-dependent changes in glucose levels at the 0 minutes time point showed a slight difference between P/P and R/R, where R/R showed an increase in glucose levels at 0 minutes primarily between 10 and 20 weeks, while this increase was observed between 20 and 40 weeks for P/P (figure 2a, p = 0.04 for age-by-genotype interaction). Ageing-related changes in glucose levels at other time points were comparable between P/P and R/R. No significant differences in glucose levels between P/P and R/R independent of ageing were observed. Wild type mice showed a smaller ageing-related increase in glucose 15 minutes after glucose administration compared to P/P and R/R carriers (both p = 0.02 for age-by-genotype interaction). Differences between genotypes at specific age (ageing independent) were analyzed using a t-test (supplementary figure 1). Wild type mice had a higher 15 minutes glucose level compared to P/P and R/R carriers at 10 weeks (p = 0.02 and p = 0.0004 respectively). Furthermore, at 10 weeks P/P carriers showed slightly higher glucose levels at 30 minutes (p = 0.04).
Oral glucose tolerance test.
(A). 0 minutes, p = 0.04 for age-genotype interaction (P/P vs R/R). (B). 15 minutes, p = 0.02 for age-by-genotype interaction (wild type vs P/P and wilt type vs R/R). (C). 30 minutes. (D). 60 minutes. (E). 120 minutes. Estimated means were generated with generalized estimating equations. Error bars represent 95% confidence intervals. Connected line for wild type mice is dashed.
In order to assess differences in body composition we performed a dual-energy x-ray absorbance (DEXA) scan on a random selection of 10 mice at 11 weeks and on the remaining 10 mice at 41 weeks of age. Changes during ageing were compared between the genotypes using a general linear model with an age-by-genotype interaction (figure 3). No ageing-related differences in lean mass and fat mass were observed between P/P and R/R mice. Fat mass showed slightly less increase during ageing in wild type mice compared to the other genotypes during ageing (figure 3b, p = 0.02 for an age-by-genotype interaction). Differences in body composition independent of ageing were assessed by a t-test. At 41 weeks of age lean mass and fat mass were significantly lower in wild type mice compared to P/P carriers (p = 0.04 and p = 0.03 respectively).
Directly after DXA scan, mice were sacrificed and fat depots were collected during necropsy and weighted as a measure of fat distribution. Ageing-dependent changes in fat depot weight (subcutaneous, mesenterial, perirenal, epididymal and interscapular fat) were compared between genotypes using a general linear model with age-by-genotype interaction after normalization for bodyweight (figure 4). Mesenterial fat showed a trend towards less increase during ageing in P/P compared to R/R mice, but this did not reach statistical significance (p = 0.08 for age-by-genotype interaction). Furthermore, P/P mice showed a weak trend towards the most modest increase in perirenal and epididymal fat depots, but this was far from significant (p = 0.40 and p = 0.29 respectively for age-by-genotype interaction). Differences in fat distribution independent of ageing were assessed by t-test. Mesenterial fat was increased at 11 weeks of age in P/P compared to R/R and wild type mice (p = 0.02 and p = 0.002 respectively).
Distribution of fat.
(A). Posterior subcutaneous fat depot. (B). Anterior subcutaneous fat depot. (C). Perirenal fat depot. (D). Mesenterial fat depot. (E). Interscapular fat depot. (F): Epididymal fat depot. Weight of fat depots are expressed in percentage (weight fat depot/bodyweight * 100%). Estimated means were generated with a general linear model. Error bars represent 95% confidence intervals. No significant ageing-dependent changes using an age-by-genotype interaction in a general linear model. * p < 0.05 using a t-test.
Discussion
Here, we showed that differences in development of waist circumference and glucose levels between P/P and R/R carriers during ageing as observed in humans8, could not be replicated convincingly in the mouse model. We did observe a borderline significant delay in fasting glucose levels P/P carrying mice during ageing but this was only at base line (0 minutes) before glucose load. Also a delayed increase in mesenterial fat depot was observed in P/P carrying mice, but this was a very small difference and not significant and is therefore interpreted as not relevant. Our results argue against the hypothesis that prevention of ageing-related increases in these parameters is involved in the longevity phenotype observed in human P/P carriers8. Because waist circumference is not a valid parameter in mice, we measured body composition (lean versus fat mass) and fat distribution. Differences between wild type mice compared to P/P and R/R carrying mice might be caused by different breeding strategy, since P/P and R/R mice were generated by heterozygous breeding pairs, which cannot generate wild type mice. This was inevitable because our major aim was to generate P/P and R/R mice from the same breeding pairs. Blood pressure measurements where beyond the scope of this study. Therefore, we cannot exclude effects of the rs1042522 polymorphism on blood pressure in the mouse model. The same holds for studies using high fat diets. An association with metabolism related quantitative traits was also not observed by Gaulton and colleagues9. However, this might not necessarily argue against previous findings of Reiling et al8 since the latter was a longitudinal study, while Gaulton et al used a cross sectional approach.
One explanation for the lack of confirmation of the observations made in humans is that the mice were too young at time of measurements. However, ageing-related changes in glucose levels and waist circumference observed in humans were independent of age at start of the study. Therefore, in young animals an effect on glucose levels and body composition was expected. Effect sizes observed in humans were small. If the effect sizes are as small in mice as they were in the human study, the experiments described here were underpowered to detect such effects. However, it was expected that effect sizes would have been larger in genetically identical mice in a controlled environment compared to genetic highly heterogeneous humans with diverse lifestyles. If this expectation was not correct, it might explain the few borderline significant results we observed. Gender specific differences are not expected since this was not observed in the human study. An explanation of our lack of confirmation of previous findings in humans, is that rs1042522 is not a true causal variant, but reflects the effects of another SNP, which is causal. In fact, several SNPs are in linkage disequilibrium10 with minor allele frequencies between 0.13 and 0.36. Although previous studies have shown that rs1042522 is a causal variant for other parameters7,11,12, we cannot exclude that it is not causal in relation to glucose metabolism and fat distribution. Another possibility, which cannot be excluded, is that the observations made in humans are false positive findings. Finally, the time span of this study could have been too short to detect changes in glucose tolerance and body composition. By all means, it is clear from our data that the here described mouse model is not a suitable model for analyses of disturbances in glucose metabolism and body composition using the strategy applied in this study.
Methods
Generation and maintenance of animals
Approval for animal experiments was provided by the animal experimental committee of the Leiden University Medical Center. All experiments were performed in accordance with institutional guidelines and regulations.
Pro/pro (P/P) and arg/arg (R/R) carriers were generated by crossing heterozygous (P/R) mice in pure C57BL6/J background, using the same breeding pairs for both cohorts. Wild type mice from our own animal breeding facility (normal mouse Trp53) in C57BL6/J background were used as reference. Only male mice were included (n = 20 per genotype) and were maintained on a standard chow diet (Rat and Mouse no3 Breeding diet, Special Diet Services, Essex, UK) during the complete study.
Oral glucose tolerance test
To test glucose homeostasis we performed oral glucose tolerance tests (ogtt). At 10 weeks of age, all mice were overnight fasted and subjected to an ogtt. Glucose levels were measured after oral glucose administration (2 g/kg) at 0, 15, 30, 60 and 120 minutes. This strategy was repeated at 20 and 40 weeks of age. Ageing-related changes of glucose measurements at the independent time points were compared using an age-by-genotype interaction in generalized estimating equations. We have chosen this approach because this is a very comparable strategy to that used in the human study, in which ageing-dependent changes in glucose levels at 30 and 60 minutes were observed8. Glucose dynamics at independent ages are shown in supplementary figure 1.
Dual-energy x-ray absorbance and necropsy
At 11 weeks of age (1 week after the first ogtt), 10 mice of each genotype were randomly selected and subjected to a dual-energy x-ray absorbance (DEXA) scan (pDEXA, Norland Stratec Medizintechnik GmbH, Birkenfeld, Germany). The remaining 10 animals underwent a DEXA scan at 41 weeks (1 week after the third ogtt). Lean and fat mass were determined with the software supplied by the manufacturer. Changes during ageing were compared between the genotypes using a general linear model with an age-by-genotype interaction. Directly after the DEXA scan, animals were sacrificed and fat depots were excised and weighted. The next fat depots were excised: posterior (hind body) and anterior (front body) subcutaneous, mesenterial (intestines), perirenal (kidney), epididymal (testis) and interscapular (between shoulder blades) fat. In order to analyze differences independent of ageing, t-tests were performed.
Statistics
In order to make this study as much comparable as possible to the human study, we focused on genotype-by-age interactions, as a measure of changes in phenotype during ageing using general estimating equations (for repeated measurements in the same mice, ogtt analyses) and general linear models (for repeated measurements in different mice, DEXA scan and necropsies). For ease of interpretation, we also analyzed time independent changes using t-tests). All mentioned statistics were performed using IBM SPSS statistics 19 (SPSS, Inc. and IBM company, New York, USA).
References
Ko, L. J. & Prives, C. p53: puzzle and paradigm. Genes Dev. 10, 1054–1072 (1996).
Lozano, G. & Elledge, S. J. p53 sends nucleotides to repair DNA. Nature. 404, 24–25 (2000).
Orsted, D. D., Bojesen, S. E., Tybjaerg-Hansen, A. & Nordestgaard, B. G. Tumor suppressor p53 Arg72Pro polymorphism and longevity, cancer survival and risk of cancer in the general population. J. Exp. Med. 204, 1295–1301 (2007).
van Heemst, D. et al. Variation in the human TP53 gene affects old age survival and cancer mortality. Exp. Gerontol. 40, 11–15 (2005).
Whibley, C., Pharoah, P. D. & Hollstein, M. p53 polymorphisms: cancer implications. Nat. Rev. Cancer. 9, 95–107 (2009).
Thomas, M. et al. Two polymorphic variants of wild-type p53 differ biochemically and biologically. Mol. Cell Biol. 19, 1092–1100 (1999).
Zhu, F. et al. Mouse models for the p53 R72P polymorphism mimic human phenotypes. Cancer Res. 70, 5851–5859 (2010).
Reiling, E. et al. Codon 72 polymorphism (rs1042522) of TP53 is associated with changes in diastolic blood pressure over time. European journal of human genetics: EJHG 20, 696–700, 10.1038/ejhg.2011.240 (2012).
Gaulton, K. J. et al. Comprehensive association study of type 2 diabetes and related quantitative traits with 222 candidate genes. Diabetes 57, 3136–3144, 10.2337/db07-1731 (2008).
Pangilinan, F. et al. Construction of a high resolution linkage disequilibrium map to evaluate common genetic variation in TP53 and neural tube defect risk in an Irish population. American journal of medical genetics. Part A 146A, 2617–2625, 10.1002/ajmg.a.32504 (2008).
Dumont, P., Leu, J. I., Della Pietra, A. C., 3rd, George, D. L. & Murphy, M. The codon 72 polymorphic variants of p53 have markedly different apoptotic potential. Nat Genet 33, 357–365, 10.1038/ng1093 (2003).
Thomas, M. et al. Two polymorphic variants of wild-type p53 differ biochemically and biologically. Mol Cell Biol 19, 1092–1100 (1999).
Acknowledgements
This study was partly financed by a NIH/NIEHS grant (1UO ES11044). Sandra Imholz is acknowledged for assistance during necropsies.
Author information
Authors and Affiliations
Contributions
E.R., M.D. and H.v.S. designed the experiments. E.S. performed animal experiments with assistance of S.J.W.N., I.R. and E.R. A.C.M.P. and S.A.A.v.d.B. provided access to and technical support with the DXA scanner. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary figure 1. Glucose dynamics during ogtt at independent ages.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Reiling, E., Speksnijder, E., Pronk, A. et al. Human TP53 polymorphism (rs1042522) modelled in mouse does not affect glucose metabolism and body composition. Sci Rep 4, 4091 (2014). https://doi.org/10.1038/srep04091
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep04091
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.