Abstract
Cryopreservation methods using liquid nitrogen (LN2) for gametes and embryos are prevalent in mammalian artificial reproduction. However, the pregnancy rate from frozen embryos has not improved over the past two decades because freeze–thawing causes significant damage. The strict regulation of transportation of LN2 containers by airlines also limits exchange between breeders. In this article, we introduce a medium that enabled bovine embryos to be held for up to 7 days at 4°C. A pregnancy rate of 75% (24/32) was obtained for embryos held for 7 days in this medium and transferred to primed recipients. Its constituents were medium 199, foetal bovine serum and HEPES for buffering. This technique will enable LN2-free storage and air transportation of embryos provided transplantation to recipients can be completed within 7 days.
Similar content being viewed by others
Introduction
Pregnancy rates after artificial insemination (AI) are as low as 5–10% on dairy farms in the hot season1,2, because bovine embryos are sensitive to the heat stress3,4, particularly between fertilization and day 7 of gestation. The technique of embryo transfer (ET) can eliminate such heat stress5,6,7, leading to markedly improved pregnancy rates. Bovine embryos produced in vivo are usually collected from superovulated cows 7 days after oestrus and transferred to recipient cows within 1 day. If the recipient cow is not at the desired phase of its oestrous cycle, the collected embryos are usually cryopreserved in liquid nitrogen (LN2) until the recipient is ready. However, this is not appropriate for short-term storage because freezing with LN2 inevitably causes cryodamage to the cells.
Many cell preservation media that are effective at hypothermic (+4°C)8,9,10 and cryogenic (−196°C)11,12,13 temperatures have been developed since the 1940s for the safe storage and transport of embryos. The latter approach using LN2 has become dominant, especially in the field of artificial reproductive technology. However, the worldwide pregnancy rate using cryopreserved mammalian embryos has not been improved over the past two decades14, probably because the freeze-thawing processes cause significant damage. Thus, during thawing, dense cryoprotectants should be diluted very carefully to avoid rupturing the embryo by osmotic shock. Moreover, the transportation of LN2 containers is strictly regulated by the International Air Transport Association (IATA)15, so alternative ways have to be used for moving embryos, e.g., by train, truck or ship. Therefore, it is relevant to re-examine the feasibility of short-term non-freezing methods of preservation and to investigate whether such methods could be applied to produce embryos with high vitality that are scheduled for transfer into recipients within several days. Therefore, it is necessary to develop a medium that maintains the viability of embryos at 4°C.
Hypothermic storage of embryos was first explored in the 1940s8,9. Phosphate-buffered saline (PBS) containing 10% (v/v) foetal bovine serum (FBS), was able to extend the preservation time of chilled bovine embryos to 1–3 days at most, but pregnancy rates were less than 50%16. Clearly, a 3-day limit would be insufficient to allow transportation between distant cattle breeders. If embryos could be preserved without freezing for periods as long as one week, they could be stored in a home refrigerator, or on ice and they could also be transported by air without using LN2 containers. This would enhance the efficiency of cattle production, especially dairy cattle. The aim of this study was to develop and evaluate an efficient medium able to extend the lifespan of embryos under hypothermic conditions.
Results
Optimization of the FBS concentration and medium base
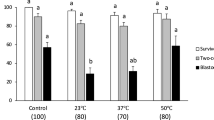
To determine the optimal concentration of FBS for hypothermic preservation, we stored blastocysts for 72 h at 4°C in PBS supplemented with FBS at a range of concentrations (0%, 5%, 20%, 50% and 100%). One hundred blastocysts produced by in vitro fertilization (IVF) were randomly assigned to five experimental groups. The viability and hatching rates of the embryos after hypothermic storage at 4°C for 72 h were higher for embryos stored in PBS with 50% FBS than for embryos stored at other FBS concentrations (Table 1).
Bovine embryos have generally been stored in PBS at hypothermic temperatures9, although one study preserved goat preantral follicles in medium 199 at 4, 20 and 39°C17. In addition, Yang and Honaramooz18 reported that Leibovitz L15 medium (L15) was effective for the hypothermic preservation of porcine testicular cells. We therefore examined the suitability of different basal media for hypothermic preservation. Ninety IVF-derived blastocysts were assigned randomly to three experimental groups. The viability rates of bovine IVF embryos after hypothermic storage for 72 h were significantly higher in embryos stored in medium 199 plus 50% FBS, compared with PBS plus 50% FBS and L15 plus 50% FBS (P < 0.05; Table 2). We also examined the effect of other basal media (DMEM, SMEM, MEMalpha and RPMI) for hypothermic preservation. The viability rates of the embryos in these media were lower (0–16.7%) than in PBS and L15. Thus, medium 199 supplemented with 50% FBS was judged suitable as a basal medium for hypothermic preservation of bovine embryos.
Effect of HEPES on hypothermic preservation
To evaluate the effect of HEPES, hypothermic preservation of embryos was performed in medium 199 plus 50% FBS, supplemented with various concentration of HEPES (0, 12.5, 25.0, 50.0 and 100 mM). We used 151 poor-quality embryos produced in vivo and assigned them randomly to five experimental groups. These embryos were collected from Japanese black beef cattle (Wagyu) donors. The viability and hatching rates of the embryos after hypothermic storage for 168 h were significantly increased for medium 199 plus 50% FBS supplemented with 12.5–50.0 mM HEPES compared with medium without HEPES (P < 0.05, Table 3). We also assessed the viabilities of embryos stored in media supplemented with other Good's buffers (25.0 mM each of TES, PIPES, MOPS and EPPS). Although the viabilities in PIPES and MOPS were significantly higher than in the medium lacking buffers (P < 0.05), the results were inferior to storage using HEPES (Table 4).
Evaluation of pregnancy rates and birth rates
To examine the pregnancy rate of chilled embryos, 32 high-quality Wagyu embryos produced in vivo were stored at 4°C for 168 h in 25 mM HEPES medium 199 plus 50% FBS. Because all of the chilled embryos stored for 168 h showed good morphology (Figure 1), they were transferred to recipient heifers. The pregnancy rate of the chilled embryos was extremely high: no embryonic death was observed between pregnancy days 30 and 60 (Table 5) and no abortions in late gestation were observed. The pregnancy rates for the chilled embryos were similar to those for fresh and conventionally frozen embryos (Table 5). The rates of producing live calves from embryos stored at 4°C (79.2%, 19/24) were the same as those of fresh and frozen embryos (80.0%, 20/25; the remaining recipient heifers are still pregnant). Approximately half (53.1%, 26/49) of the calves born to these heifers were delivered unassisted, whereas 20.4% (10/49) were classified as suffering severe dystocia and died. A similar result has been reported by Lombard et al.19. We did not observe malformation or large offspring syndrome (LOS) in the dead calves. There were no significant differences in the mean (±SEM) birth weight of live calves from chilled embryos (34.2 ± 6.4 kg; n = 19) versus non-chilled embryos (38.3 ± 4.9 kg; n = 20) or gestation lengths (chilled embryos 289.2 ± 4.9 days, non-chilled embryos 288.6 ± 5.7 days; P > 0.05). All the live calves stood up when born and suckled and appeared to be normal and healthy (Figure 2).
Discussion
Little is known about the hypothermic preservation of mammalian embryos, particularly bovine embryos because LN2 cryopreservation has generally been applied to embryo storage for cattle production. Serum additives or bovine serum albumin (BSA)-based preparations are widely used as supplements for cryopreservation medium, despite the undefined nature of serum composition20,21. These additives were reported to protect cell membranes during the freezing process22. Although it was shown that bovine embryos could be stored in PBS containing 10% bovine serum at hypothermic temperatures16, the optimal concentration of serum has not been evaluated. Therefore, we determined the optimal concentrations of serum or BSA in stock solution during “non-freezing” chilled preservation. In our preliminary tests, the survival rate of bovine embryos after hypothermic storage at 4°C for 72 h was higher in embryos stored in medium with 50% FBS compared with low (4 mg/ml) or high (10 mg/ml) concentrations of BSA. Our results suggest that the concentration of FBS in the medium affects the survival rate of embryos after chilling preservation; the optimal concentration in the present study was 50%.
Serum contains a wide variety of substances, including energy substrates, growth factors, cytokines and hormones23. It also contains amino acids that play important roles as osmolytes and pH buffers24. On the basis of our observations, it could be assumed that a high serum concentration in the hypothermic medium enhanced embryo viability following chilling preservation. However, the addition of serum to culture medium for embryos causes alterations in mitochondrial structure25, which could impair the ability of bovine embryos to metabolize lipids26. Moreover, the LOS has been described in late ruminant gestation and causes significant numbers of perinatal deaths following culture of IVF-derived embryos in the presence of serum27. LOS is characterized by a multitude of pathologies, including high birth weights (>50 kg), increased gestation length, frequent dystocia and elevated abortion rates. Although the artificial-dormancy medium that we developed contains a high concentration of serum in the form of FBS, the rates of abortion, dystocia and full-term births of embryos stored at 4°C were the same as those of fresh and conventionally frozen embryos, as were their birth weights and gestation lengths. During chilling preservation, the metabolism of embryos is inhibited and the embryos are dormant. Thus, these results suggest that the presence of serum during chilling preservation does not inevitably affect the later health of offspring during gestation.
The most commonly used basal medium for hypothermic preservation of bovine and sheep embryos is PBS16,28,29. We examined effect of FBS on preservation when PBS, medium 199 and L15 were used as basal media. Although all three media containing FBS showed more than 50% viability rates after preservation at 4°C for 72 h (Table 2), the best preservation result was obtained with medium 199. It was reported that PBS and L15 with phosphate buffers were more efficient than bicarbonate-buffered medium 199 or DMEM for hypothermic storage18. Furthermore, medium 199 was found to be unsuitable for the short-term preservation of porcine oocytes at ambient temperature30. Our results are inconsistent with these observations and indicated that medium 199 was superior. This suggests that neither phosphate nor bicarbonate buffers are essential for the ‘chilled’ storage of embryos. Indeed, our preliminary experiments showed that both medium 199 and PBS preserved the viabilities of bovine embryos stored for 72 h at ambient temperature. Thus, medium 199 would also be expected to maintain embryonic cells in a chilled environment.
We next focused on the concentration of HEPES in hypothermic medium. The presence of HEPES dramatically improved the viability of bovine embryos stored at 4°C in medium 199 plus 50% FBS. HEPES is one of Good's buffers, widely used in cell culture because it is better at maintaining physiological pH despite changes in CO2 concentration. The optimal physiological pH for mammalian cells is usually considered to be pH 6.8–7.2. In this study, although the pH range of media buffered with HEPES at the end of the preservation period was 6.9–7.4, that of the medium without HEPES was slightly higher (7.7, Table 3). This suggests that an elevation of pH in hypothermic medium impairs the viability of chilled embryos. Therefore, we examined whether Tris, another widely used buffering agent, could improve the viability of chilled bovine embryos. The viability after 168 h was only 10% when embryos were stored in medium 199 with 50% FBS supplemented with 20 mM of Tris, although this medium remained stable at pH 7.2–7.3 after use. Thus, HEPES has beneficial effects for embryo preservation, allowing only minor biochemical effects during hypothermic preservation.
For example, there is a possibility that HEPES might change the efflux of P-glycoprotein31. This is distributed and expressed extensively in early embryonic cells32 and is an ATP-dependent efflux pump with broad substrate specificity. It probably evolved as a defence mechanism against harmful substances. Furthermore, HEPES suppresses the action of chloride channels33. Rubinsky et al. reported that the inhibition and suppression of ion channel activities were important for chilling preservation of mammalian cells34. Therefore, our results suggest that supplementation with HEPES in medium has a beneficial influence on the plasma membrane of embryonic cells during cold storage. Although the HEPES concentrations used in incubation buffers differ greatly from laboratory to laboratory, with a range of 10–100 mM35,36, 25 mM HEPES is one of the most commonly added ingredients in biological buffer systems. Taken together, medium 199 plus 50% FBS supplemented with 25.0 mM HEPES was the most suitable for hypothermic preservation of bovine embryos. The pregnancy rate of chilled embryos stored in the artificial dormancy medium was extremely high in this study. This protocol might be useful when a recipient animal is a few days before the optimal time for ET in its oestrous cycle and this would enable direct ET, which would simplify fieldwork. This technique could enhance the efficiency of worldwide dairy cow production and might even be extended to endangered species and laboratory animals.
In conclusion, maintaining artificial dormancy of bovine embryos using a simple medium appears to be feasible. This is the first documented success of storing chilled mammalian embryos in a viable state for 1 week. However, to be of practical value, mammalian embryo preservation at hypothermic temperatures must be able to maintain viability for even longer periods.
Methods
Animal care
The Committee for Experimental Animals of Zen-noh Embryo Transfer Center approved all animal procedures in this study. Donor cows (3–7 years old) and recipient heifers (14–18 months old) were fed the same food and water was supplied ad libitum. The herds were comprised considering body constitution and social hierarchy.
Chemicals
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise stated.
Oocyte maturation and in vitro fertilization (IVF)
The in vitro production system (maturation and IVF of oocytes and culture of embryos) used the methods reported by Ideta et al.37. Briefly, bovine ovaries were obtained at a local slaughterhouse and brought to our laboratory in sterile saline solution of 0.85% NaCl after testing the donors for bovine spongiform encephalitis infection. The cumulus–oocyte complexes (COCs) were aspirated from 2–7 mm diameter follicles. Only COCs with a compact, non-atretic cumulus oophorus and corona radiate and a homogenous ooplasm were selected for in vitro maturation. Groups of 30–40 COCs were cultured for 22 h with 700 μl of tissue culture medium 199 containing 25 mM HEPES (Invitrogen, Carlsbad, CA, USA, Cat. no. 12340) and 5% FBS (MP Biomedicals, Illkirch, France, Cat. no. 2917354) in 4-well tissue culture plates (Nunc, Nalge Nunc International, Roskilde, Denmark) under sterile paraffin oil (Nacalai Tesque, Kyoto, Japan) at 38.5°C under 5% CO2 in air with high humidity37.
IVF was performed using IVF100 medium (Research Institute for the Functional Peptides, Yamagata, Japan) according to the manufacturer's instructions. Briefly, frozen–thawed spermatozoa were washed twice by centrifugation (600 g for 10 min) in IVF100 medium. The sperm pellet was diluted with IVF100 to prepare a final cell concentration of 5 × 106/ml. After maturation, COCs were washed three times with IVF100 and then transferred into droplets of sperm cell suspension (30 oocytes/100 μl droplet under sterile paraffin oil). These were incubated for 6 h at 38.5°C under 5% CO2 in air with high humidity.
IVF-derived embryos were co-cultured with a bovine cumulus cell monolayer in 700 μl of CR1aa medium38 with 5% FBS for 7 days in 4-well tissue culture plates under sterile paraffin oil at 38.5°C under 5% CO2 in air with high humidity. On day 7, grades 1 to 2 (high-quality) blastocysts according to the International Embryo Transfer Society (IETS) classification39 were used for further study.
Collection of in vivo fertilized embryos
Embryos produced in vivo by artificial insemination were collected from superovulated Wagyu cows. Briefly, oestrus was synchronized with a progesterone-releasing intravaginal device (PRID, ASKA Pharmaceutical Co., Ltd., Tokyo, Japan) inserted into the vagina on random days of the oestrus cycle for 9 days. Two days before removal of the PRID, prostaglandin F2alpha (PGF2α) (2 ml, Resipron-C containing 0.25 mg/ml Cloprostenol, ASKA Pharmaceutical Co., Ltd.,) was administered to the cows by intramuscular (i.m.) injection40. The superovulatory treatment began in the mid-luteal phase of the oestrus cycle (days 8–10) and consisted of six decreasing i.m. doses of follicle-stimulating hormone (FSH, total of 20 Armour units, Antrin R-10, Kawasaki-Mitaka, Kanagawa, Japan) for 3 days with treatment twice daily. PGF2α was injected i.m. at the fifth FSH treatment. In addition to this superovulatory treatment, 1 ml of gonadotrophin-releasing hormone analogue (Conceral injection containing 50 μg/ml fertirelin acetate, Schering-Plough Animal Health K. K., Tokyo, Japan) was administered i.m. 48 h after the PGF2α treatment to induce ovulation of the growing follicles. The cows were then artificially inseminated with frozen-thawed semen. On day 7 (day 0 = day of oestrus), a multi-eye 16-French embryo collection catheter (Nipro Corp., Osaka, Japan) was non-surgically inserted into the cows and embryos were collected from the uterus by uterine flushing. The flushing medium consisted of lactated Ringer's solution (Terumo Corp., Tokyo, Japan) supplemented with 0.1% FBS. Grades 1 to 2 (high quality) and 2.5 (poor quality) embryos were used in this study according to the IETS classification39.
Hypothermic storage of bovine embryos
Bovine embryos were washed three times in each test medium (see below) and loaded into a plastic straw (1/4 cm3 clear straw, Pets-Inc., Canton, TX, USA). Then, after 20 min at room temperature, the embryos were stored at refrigeration temperature (4 ± 1°C, Medicool, Sanyo, Osaka, Japan) for 72 or 168 h.
Optimizing the concentrations of FBS, medium base and HEPES
IVF-derived blastocysts were stored for 72 h at 4°C in a plastic straw containing PBS (Invitrogen, Cat. no. 21300) plus 0%, 5%, 20% 50% or 100% of FBS (MP Biomedicals, Cat. no. 2917354). The following mixtures were loaded into the straw from left to right: PBS with 5% FBS, an air bubble, test solution (PBS with 0–100% FBS) containing 2–5 bovine embryos, another air bubble and more PBS with 5% FBS. The straw was sealed hermetically. One mg/ml polyvinyl alcohol was added to the PBS lacking FBS. The loaded straws were placed in a refrigerator set to 4°C. After 72 h, the stored embryos were transferred from the straw to 3 ml of PBS with 5% FBS and washed three times with the same medium. The embryos were incubated with PBS plus 5% FBS for 15 min at room temperature, then transferred to CR1aa medium38 supplemented with 5% FBS for 48 h at 38.5°C under 5% CO2 in air with high humidity. The viability and hatching rates of the embryos were estimated at the end of the culture period. Briefly, embryos that appeared dark and shrunken with no cell proliferation or cellular integrity were judged to have degenerated. Viable embryos that had made a clear breach of the zona pellucida with the trophectoderm were classified as hatching blastocysts.
Although bovine embryos have been stored in medium such as PBS at hypothermic temperatures16, medium 199 can be used in preserving goat preantral follicles at 4, 20 and 39°C17. Furthermore, Yang and Honaramooz18 reported that L15 could be used effectively as a defined medium for the hypothermic preservation of porcine testicular cells. Next, IVF-derived blastocysts were stored at 4°C in a plastic straw in one of the following three media from Invitrogen: PBS (Cat. no. 21300), medium 199 (Cat. no. 12340) or L15 (Cat. no. 41300), all with 50% FBS for 72 h. We also examined the effects of four other basal media from Invitrogen: DMEM (Cat. no. 11885), SMEM (Cat. no. 11385), MEMalpha (Cat. no. 12571) and RPMI (Cat. no. 11875) for hypothermic preservation. Following storage, the embryos were cultured in vitro for 48 h and assessed for viability and hatching rate as above.
Poor-quality Wagyu blastocysts produced in vivo were stored at 4°C in a plastic straw in medium 199 without HEPES (Invitrogen, Cat. no. 11150) plus 50% FBS supplemented with various concentrations of HEPES (0, 12.5, 25.0, 50.0 and 100 mM; Dojindo, Kumamoto, Japan, Cat. no. 348-01372) for 168 h. We also examined the effect of Good's buffers—25.0 mM each of TES, PIPES, MOPS and EPPS—and 20 mM Tris41 buffer in addition to HEPES. Following storage, the chilled embryos were cultured in vitro for 48 h and assessed for viability and hatching rate as above.
Evaluation of pregnancy rates using chilled embryos and live births
High-quality Wagyu embryos (morula to blastocyst stages) produced in vivo were stored for 168 h at 4°C in a plastic straw in medium 199 plus 50% FBS supplemented with 25 mM HEPES (hypothermic medium). The following mixtures were loaded into the straw from left to right: hypothermic medium, an air bubble, hypothermic medium containing one bovine embryo, another air bubble and more hypothermic medium. The straw was sealed hermetically (Figure 3). The loaded straws were placed in a refrigerator set to 4°C. After 168 h, the stored embryos were transferred into PBS with 5% FBS and washed three times in the same medium. We observed the morphology of the chilled embryos using light microscopy (Leica Wild M3Z, Leica, Wetzlar, Germany) and determined whether ET would be possible. The chilled embryos were reloaded into plastic straws with the washing medium and transferred non-surgically into Holstein heifers (one embryo per recipient) to the uterine horn ipsilateral to the existing corpus luteum using an ET device (mo-No.1, Misawa Medical Industry Co., Ltd, Tokyo, Japan) on days 6–8 of the oestrous cycle (day of oestrus = day 0). Pregnancy was determined by real-time B-mode ultrasonography (Convex scanner HS-1500, Honda electronics Co. Ltd., Toyohashi, Japan) on days 30 and 60 of gestation. Non-chilled, fresh and frozen42 embryos were used as controls. In this study, calves were delivered spontaneously without induction of parturition.
Statistics
Data were analysed using StatView software (version 5, SAS Institute, Cary, NC, USA). The statistical significance of any differences between treatments was assessed by Chi-squared (viability, hatching and pregnancy rates for chilled embryos) and Student's t tests (calf birth-weight and gestation length) and P < 0.05 was taken as significant.
References
Badinga, L., Collier, R. J., Thatcher, W. W. & Wilcox, C. J. Effects of climate and management factors on conception rate in dairy cattle in subtropical environments. J Dairy Sci 68, 78–85 (1985).
Burke, J. M. et al. Evaluation of timed insemination using a gonadotropin-releasing hormone agonist in lactating dairy cows. J Dairy Sci 79, 1385–1393 (1996).
Al-Katanani, Y. M., Paula-Lopes, F. F. & Hansen, P. J. Effect of season and exposure to heat stress on oocyte quality of Holstein cows. J Dairy Sci 85, 390–396 (2002).
Zeron, Y. et al. Seasonal changes in bovine fertility: relation to developmental competence of oocytes, membrane properties and fatty acid composition of follicles. Reproduction 121, 447–457 (2001).
Putney, D. J., Drost, M. & Thatcher, W. W. Influence of summer heat stress on pregnancy rates of lactating dairy cattle following embryo transfer or artificial insemination. Theriogenology 31, 765–778 (1989).
Drost, M. et al. Conception rates after artificial insemination or embryo transfer in lactating dairy cows during summer in Florida. Theriogenology 52, 1161–1167 (1999).
Ambrose, J. D. et al. Efficacy of timed embryo transfer with fresh and frozen in vitro produced embryos to increase pregnancy rates in heat-stressed dairy cattle. J Dairy Sci 82, 2369–2379 (1999).
Chang, M. C. Normal development of fertilized rabbit ova stored at low temperature for several days. Nature 159, 602 (1947).
Chang, M. C. The effects of low temperature on fertilized rabbit ova in vitro and the normal development of ova kept at low temperature for several days. J Gen Physiol 31, 385–410 (1948).
Hafez, E. S. E. Storage of rabbit ova in gelled media at 10°C. J Reprod Fertil 2, 163 (1961).
Whittingham, D. G. Survival of mouse embryos after freezing and thawing. Nature 233, 125–126 (1971).
Whittingham, D. G., Leibo, S. P. & Mazur, P. Survival of mouse embryos frozen to −196 degrees and −269 degrees C. Science 178, 411–414 (1972).
Wilmut, I. & Rowson, L. E. A. Experiments on the low-temperature preservation of cow embryos. Vet Res 92, 686–690 (1973).
Nishigai, M. The development and prevalence of the transfer technique for frozen-thawed embryos of Japanese black beef cattle in Tochigi prefecture. J Reprod Dev 49, 23–36 (2003).
IATA dangerous goods regulations manual 54th edition (International Air Transport Association, Product Code: BK-IATA13, 2013).
Lindner, G. M. & Ellis, D. E. Refrigeration of bovine embryos. Theriogenology 23, 202 (1985).
Costa, S. H. F. et al. Preservation of goat preantral follicles enclosed in ovarian tissue in saline or TCM 199 solutions. Small Rumin Res 58, 189–193 (2005).
Yang, Y. & Honaramooz, A. Effects of medium and hypothermic temperatures on preservation of isolated porcine testis cells. Reprod Fertil Dev 22, 523–532 (2010).
Lombard, J. E., Garry, F. B., Tomlinson, S. M. & Garber, L. P. Impacts of dystocia on health and survival of dairy calves. J Dairy Sci 90, 1751–1760 (2007).
Tominaga, K., Iwaki, F. & Hochi, S. Conventional freezing of in vitro-produced and biopsied bovine blastocysts in the presence of a low concentration of glycerol and sucrose. J Reprod Dev 53, 443–447 (2007).
Whittingham, D. G. The viability of frozen-thawed mouse blastocysts. J Reprod Fert 37, 159–162 (1974).
Lane, M., Maybach, J. M., Hooper, K., Hasler, J. P. & Gardner, D. K. Cryo-survival and development of bovine blastocysts are enhanced by culture with recombinant albumin and hyaluronan. Mol Reprod Dev 64, 70–78 (2003).
Shah, G. Why do we still use serum in the production of biopharmaceuticals? Dev Biol Stand 99, 17–22 (1999).
Bavister, B. D. Culture of preimplantation embryos: facts and artifacts. Hum Reprod Update 1, 91–148 (1995).
Dorland, M., Gardner, D. K. & Trounson, A. O. Serum in SOF causes mitochondrial degeneration in ovine embryos. J Reprod Fertil 13, 70 (1994).
Crosier, A. E. et al. Ultrastructural morphometry of bovine compact morulae produced in vivo or in vitro. Biol Reprod 62, 1459–1465 (2000).
Behboodi, E. et al. Birth of large calves that developed from in vitro-derived embryos. Theriogenology 44, 227–232 (1995).
Trounson, A. O., Willadsen, S. M. & Rowson, L. E. A. The influence of in-vitro culture and cooling on the survival and development of cow embryos. J Reprod Fert 47, 367–370 (1976).
Baguisi, A. et al. Hypothermic storage of sheep embryos with antifreeze proteins: Development in vitro and in vivo. Theriogenology 48, 1017–1024 (1997).
Yang, C. R. et al. Short-term preservation of porcine oocytes in ambient temperature: novel approaches. PLoS One 5, e14241 (2010).
Luo, S. et al. Effect of HEPES buffer on the uptake and transport of P-glycoprotein substrates and large neutral amino acids. Mol Pharm 7, 412–420 (2010).
Elbling, L., Berger, W., Rehberger, A., Waldhör, T. & Micksche, M. P-glycoprotein regulates chemosensitivity in early developmental stages of the mouse. FASEB J 7, 499–1506 (1993).
Yamamoto, D. & Suzuki, N. Blockage of chloride channels by HEPES buffer. Proc R Soc Lond B Biol Sci 230, 93–100 (1987).
Rubinsky, B. et al. Inhibition of Ca++ and K+ currents by “antifreeze” proteins. Am J Physiol 262, 542–545 (1992).
Akiba, T., Rocco, V. K. & Warnock, D. G. Parallel adaptation of the rabbit renal cortical sodium/proton antiporter and sodium/bicarbonate cotransporter in metabolic acidosis and alkalosis. J Clin Invest 80, 308–315 (1987).
McCarthy, J. E., Ferguson, S. J. & Kell, D. B. Estimation with an ion-selective electrode of the membrane potential in cells of Paracoccus denitrificans from the uptake of the butyltriphenylphosphonium cation during aerobic and anaerobic respiration. Biochem J 196, 311–321 (1981).
Ideta, A., Urakawa, M., Aoyagi, Y. & Saeki, K. Early development in utero of bovine nuclear transfer embryos using early G1 and G0 phase cells. Cloning Stem Cells 9, 571–580 (2007).
Rosenkrans, C. F., Zeng, G. Q., McNamara, G. T., Schoff, P. K. & First, N. L. Development of bovine embryos in vitro as affected by energy substrates. Biol Reprod 49, 459–462 (1993).
IETS. Manual of the international embryo transfer society. In: Stringfellow, D. A., Seidel, S. M., editors. Savoy, Illinois, USA: International Embryo Transfer Society (1998).
Aoyagi, Y. et al. Effects of a combination of a PRID, PGF2α and eCG, on estrus synchronization and pregnancy rate following embryo transfer in Holstein heifers. Reprod Fertil Dev 19, 220 (2007).
Abeydeera, L. R. & Day, B. N. Fertilization and subsequent development in vitro of pig oocytes inseminated in a modified Tris-buffered medium with frozen-thawed ejaculated spermatozoa. Biol Reprod 57, 729–734 (1997).
Aoyagi, Y. et al. Effect of lipid rich bovine serum albumin on direct transfer of frozen-thawed bovine embryo. Theriogenology 45, 165 (1996).
Acknowledgements
This work was supported by the Program for Promotion of Basic and Applied Research for Innovations in Bio-oriented Industry.
Author information
Authors and Affiliations
Contributions
A.I. conceived the research, A.I., K.T., T.K. and Y.N. performed experiments and analysis, A.I., Y.A. and S.T. discussed and wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Ideta, A., Aoyagi, Y., Tsuchiya, K. et al. A simple medium enables bovine embryos to be held for seven days at 4°C. Sci Rep 3, 1173 (2013). https://doi.org/10.1038/srep01173
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep01173
This article is cited by
-
Oviduct epithelium induces interferon-tau in bovine Day-4 embryos, which generates an anti-inflammatory response in immune cells
Scientific Reports (2018)
-
Holding of bovine blastocysts at suprazero temperatures using small molecules
Scientific Reports (2017)
-
Effective vitrification and warming of porcine embryos using a pH-stable, chemically defined medium
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.