« Prev Next »
- Stabilizing selection keeps the population at one stable optimal value
- Directional selection transforms the value of a trait by increasing the frequency of individuals closer to a distant optimum
- Disruptive selection increases the frequency of large and small values of a trait at the expense of intermediate values
- Balancing selection selects the optimal compromise among several constraints
At their core, all forms of selection involve individuals with inherited differences in fitness competing within the same population. This competition is about fitness as measured by survival rates, fecundity, or some other trait that correlates with fitness. The "winner" of the competition is positively selected, and its genotype increases in frequency; on the other hand, the "loser" is negatively selected, and the frequency of its genotype decreases. Thus, negative selection and positive selection cannot be separated. To make communication easier, however, scientists talk about positive selection when the focus of a particular study is on an increase in rare variants that improve optimal fitness, and they speak of negative selection when the focus is on the removal of harmful variants.
Causes of Negative Selection

More short-term negative selection is also widespread, especially due to ecological reasons. Many structures in biology are only conditionally optimal, because they depend upon the details of other structures or circumstances to perform their function. If such other structures are within the same organism, this relationship is termed epistasis. For example, two proteins could interact epistatically in such a way that a deleterious mutation in one protein could be either compensated for or aggravated by a mutation in the other protein (Burch & Chao, 1999). Frequently, ecological circumstances also play a role in determining mutational effects. For instance, if the niche of a species stays the same, some mutations that would be beneficial in other niches will be under negative selection. If the niche changes, however, some traits that were previously under negative selection may suddenly be beneficial and have a greater fitness than the majority of the previously favored genotypes.
If environmental interactions include other rapidly evolving species, then the pressure to change may never stop, and the evolutionary optimum will always remain some steps ahead. Host-parasite interactions are a famous example of this sort of situation. Here, the host immune system evolves to recognize a special structure on the parasite and allow its removal. This in turn induces negative selection on the current form of the parasite while leading to positive selection of variants that cannot be recognized by the host. Furthermore, if such variant parasites exist, they will increase in frequency and in turn induce negative selection of the current variant of the host, which will lead to the positive selection of hosts that can again recognize the parasite, and so on. Negative (and positive) selection in such a system never rests, which is why one hypothesis describing these systems was named after the Red Queen in the book Alice in Wonderland, who famously stated that it takes a great amount of running to stay in the same place.
Effective Strength
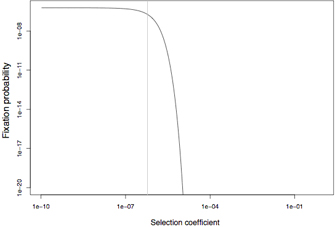
However, most selection coefficients are very small and, thus, selection as a process is often better understood by looking at the relative effective selection coefficient Nes, which is the product of the absolute selection coefficient and the effective population size Ne. A value of 1 (Nes = 1) approximately denotes a threshold: Mutations with Nes < 0.25 are fixed with probabilities that are comparable to those of neutral mutations, even if they are harmful. On the other hand, mutations in which Nes > 4 will very rarely become fixed in a population with reasonable levels of recombination. Moreover, it is unlikely that a harmful mutation with Nes exceeding a few dozen could have ever been fixed in the entire history of the known universe. Such deleterious mutations can only be fixed if they are closely linked to a very strongly advantageous mutation that will drag them to high frequencies (but, as discussed later, this reduces Ne and, thus, the rule of thumb remains valid). If harmful mutational effects are smaller than roughly Nes = 0.25, then they can pass below the "radar" of natural selection: Here, natural selection can no longer distinguish among beneficial, neutral, and harmful mutations, so it treats them all in the same way. This problem is balanced by the increasing frequency of advantageous back mutations for such sites. To apply this simple rule of thumb correctly, it is important to note that Ne usually has to be measured from DNA sequence diversity data in the wild. Ne is usually much lower than the number of all existing individuals. Also, this rule has limitations when recombination is rare or absent. In this case, the effective strength of selection is reduced, and more deleterious mutations can fix more easily. Figure 3 shows the fixation probabilities of various deleterious mutations under free recombination.
Consequences of Negative Selection
The main consequence of negative selection is the extinction of less-adapted variants. If the best-adapted variant does not change because it is at a stable local optimum, then negative selection will remove all new variants for that optimal trait.
It is important to note that negative selection can also impact molecular diversity. Consider the simple case of a population with genes that are optimally adapted to the existing constant environment. Such a setting is probably realistic for many "housekeeping" genes that ensure the proper working of the basic molecular machinery of life. Almost every mutation that happens in these genes will be deleterious, and, because mutations are the inevitable consequence of the molecular machinery that copies DNA, we can expect a substantial number of such harmful mutations. The deleterious nature of these mutations will result in their quick removal. In any real-life setting, however, an important side effect of such a removal will be the accompanying removal of linked mutations. This has important implications for the study of molecular diversity, as all neutral mutations linked to deleterious mutations will not be observed in the population and the corresponding Ne values will thus be reduced. This reduction in Ne has consequences for adaptive evolution, because the effective strength of selection for positive mutations will be reduced as well, and more advantageous mutations with small effects will be lost by chance.
If negative selection is too weak to remove harmful mutations, then deleterious mutation accumulation will occur, and a gradual decay of genomic integrity will be the result. This can lead to extinction for some species if it continues long enough; however, the resulting widespread existence of deleterious mutations in such a genome will eventually also lead to the occurrence of back mutations, which (among many other factors) can significantly contribute toward maintenance of a reasonable level of integrity in the genome of other species in the long term.
If negative selection is too strong for the whole population, extinction will occur, unless the population is rescued in time. Extinction can occur if the negative selection considered is "hard" selection, which actually reduces the number of surviving offspring that are produced. "Soft" selection (which occurs when the reproductive capacity of an organism is high enough) can also be negative, but it will lead only to competition over who will increase in frequency within the population, effectively without a reduction of the maximal number of offspring that can be produced. Thus, no extinction risk exists with soft selection.
Climate change and other habitat alterations are currently placing many species under such extreme negative selection that these species' survival is threatened. Also, mutagenic substances that are released into the environment by humans lead to a general increase in the frequency of mutations, a vast majority of which are deleterious and further increase the negative selection pressure for many populations. Thus, understanding the causes, extent, and consequences of negative selection can contribute important insights toward securing biodiversity in the long term.
References and Recommended Reading
Burch, C. L., & Chao, L. Evolution by small steps and rugged landscapes in the RNA virus phi6. Genetics 151, 921–927 (1999)
Charlesworth, D., et al. The pattern of neutral molecular variation under the background selection model. Genetics 141, 1619–1632 (1995)
Hereford, J., et al. Comparing strengths of directional selection: How strong is strong? Evolution 58, 2133–2143 (2004)
Loewe, L. Quantifying the genomic decay paradox due to Muller's ratchet in human mitochondrial DNA. Genetical Research 87, 133–159 (2006)
Sandelin, A., et al. Arrays of ultraconserved non-coding regions span the loci of key developmental genes in vertebrate genomes. BMC Genomics 5, 99 (2004)
Spielman, D., et al. Most species are not driven to extinction before genetic factors impact them. Proceedings of the National Academy of Sciences 101, 15261–15264 (2004)
Vane-Wright, D. Entomology: Butterflies at that awkward age. Nature 428, 477–480 (2004) doi:10.1038/428477a (link to article)
Westemeier, R. L., et al. Tracking the long-term decline and recovery of an isolated population. Science 282, 1695–1698 (1998) doi:10.1126/science.282.5394.1695




 Figure 1
Figure 1