Abstract
Study design:
Experimental animal model to assess ischemic spinal cord injury (SCI) following occlusion of the thoraco-abdominal aorta.
Objectives:
In the present study, we aimed to investigate the role of melatonin on SCI induced by ischemia and following reperfusion.
Setting:
Animal Research Laboratory, Inonu University, Malatya, Turkey.
Methods:
We evaluated oxidative damage and caspase-3 activity. In total, 32 adult Wistar albino rats were divided into four groups: Group 1, control (n=8); Group 2 (n=8), those subjected to ischemia/reperfusion (IR) by clamping the thoraco-abdominal aorta; Group 3 (n=8), melatonin (50 mg kg−1) treated; and Group 4 (n=8), melatonin (50 mg kg−1) followed by ischemia. All animals were kept alive for 48 h, and then spinal cord samples were removed. We assayed oxidative damage by measuring malondialdehyde (MDA), apoptosis by measuring activated caspase-3 (using immunoblots) and intrinsic antioxidative capacity by measuring reduced glutathione (GSH) levels in the spinal cord.
Results:
The results indicated a significant decrease in activity of caspase-3 in SCI animals after treatment with melatonin, as it significantly decreased the formation of MDA and decelerated the loss of GSH.
Conclusion:
This study suggested that melatonin could be an effective neuroprotective agent for treatment of SCI.
Similar content being viewed by others
Introduction
Spinal cord injury (SCI) can result in severe disability, sensory disorder, paralysis, other neurologic deficits and even death. After SCI, many cells are lost because of the injury.1 Perturbation of the Ca2+ homeostasis after injury causes defects in mitochondrial respiratory electron transport. Free radical intermediated products are continuously produced and released. These reactive oxygen species rapidly react with unsaturated and polyunsaturated fatty acid side chains of membrane lipids or with other components of the cells, thus harming the cell and generating more free radicals. The saturation of plasma membrane components by lipid peroxidation induces adverse changes of membrane properties like decreased fluidity and permeability. Malondialdehyde (MDA) is a major peroxidation product.2 An elevated concentration of MDA is one of the markers of tissue damage.3 Subsequent cell death is believed to be primarily necrotic.
Melatonin is an indoleamine, naturally produced in the pineal gland and other tissue, and it has been shown to possess several properties that may be responsible for its neuroprotective effects.4 In experimental animal models of SCI, melatonin has been found highly effective in reducing the level of lipid peroxidation following injury.5 This effect may be due to the facts that melatonin is a powerful antioxidant, and it stimulates several antioxidants including: catalase, glutathione reductase, peroxidase and superoxide dismutase. Melatonin has been shown to be highly effective in reducing oxidative damage in the central nervous system; its efficacy derives from its ability to directly scavenge a number of free radicals and to function as an indirect antioxidant.6
Melatonin is a free radical scavenger, an antioxidant and immunomodulatory agent. Antioxidant properties of melatonin are connected with its neuroprotective activity in several degenerative disorders. One of the causes of neurodegenerative damage in the nervous system in oxidative injury result from an imbalance between free radical formation and antioxidative mechanisms.7
Caspases have a pivotal role in the progression of a variety of neurological disorders. The major executioners in the apoptotic program are proteases known as caspases (cysteine-dependent aspartate-specific proteases). Caspases directly and indirectly orchestrate the morphologic changes of the cell during apoptosis. They exist as latent precursors, which, when activated, initiate the death program by destroying key components of cellular infrastructure and activating factors that mediate damage to the cells. Ischemic stroke was the first neurologic disease in which the activation of a caspase was documented.8, 9 Moreover, inhibition of caspases reduces tissue damage and allows remarkable neurologic improvement. Activation of caspases 1,3,8,9 and 11 and release of cytochrome c have been demonstrated in cerebral ischemia.10, 11, 12 Melatonin is known to stabilize membranes and thus prevents cell damage and death. As both calpain and caspase-3 activities are known to participate in cell death, the implication is that reduction of their activities by melatonin may have protected neurons in the SCI following injury.1
On the basis of these findings, in the current study, we investigated the effects of melatonin on experimental SCI-induced tissue damage in spinal cord by measuring MDA, glutathione and caspase-3 activity.
Materials and methods
Animal handling and treating with melatonin
This study was performed in the Medical Biology Research Laboratory of Inonu University, Faculty of Sciences and Arts. The experimental protocol was evaluated and approved by the Ethics Review Committee of Inonu University, Faculty of Medicine. The animals were kept at optimal (18–21 ºC) room temperature and fed a standard diet. A 12 -h light–dark cycle (0800 –2000 hours light/2001–0759 hours dark) was implemented. Free access to food and water was allowed.
The animals were anesthetized by intramuscular (i.m.) injection of ketamine (70 mg kg−1) (Ketalar, Parke-Davis, Eczacıbaşı, Istanbul, Turkey) and xylazine (5 mg kg−1) (Rompun, Bayer, Istanbul, Turkey) and allowed to breathe spontaneously. Additional doses were occasionally required during the surgery. Body temperatures were measured by rectal thermometry and maintained at 37 °C with a pad and heating lamp.
Melatonin (Sigma, St Louis, MO, USA) was dissolved in a minimum volume of ethanol (0.5 ml) and diluted to the desired concentration with physiological saline.
The spinal cord ischemia model described by Zivin and De Girolami was used in this study.13 In total, 32 rats were divided into four groups. The control group contained eight rats (C). The control group was not injured in order to compare the effects of melatonin treatment intact animals and injured animals. The second group of eight animals was subjected to IR by clamping the thoraco-abdominal aorta (IR). Another eight rats in the pre-IR group were given 50 mg kg−1 melatonin intraperitoneally (i.p.) 10 min. before the aorta was clamped for ischemia and reperfusion (Mel+IR). The remaining eight rats were given 50 mg kg−1 melatonin (Mel) but were not injured.
Ischemia–reperfusion procedure
The spinal cord ischemia model described by Zivin and DeGirolami13 was used in this study. Briefly, the thraco-abdominal aorta was clamped by a ‘Bulldog’ atraumatic infantile vascular compression clam approximately 1 cm below the renal artery. The location of the clamping corresponds to the L3 vertebrae of the animal. At the end of the occlusion period, the clamp was removed, and restoration of blood flow was visually verified. For the control study, the same group was subjected to only laparotomy without aortic compression.
Rats were killed by ketamine overdose after reperfusion. Intra-arterial perfusion was performed with a left ventricular cannula, whereas the right atrium was opened widely. During the perfusion, circulating blood was washed out with 500 ml physiological saline under 110 cm H2O pressure. The lumbosacral cords between L4 and S1 were immediately removed. The tissues were collected under a surgical microscope at × 400 magnification using a × 16 ocular and a × 25 objective (OPMI-99 microscope, Carl Zeiss Inc., Oberkochen, Germany). Spinal cord samples were labeled and placed in liquid nitrogen immediately and then transported to the laboratory for measurement of caspase 3 activity and lipid peroxidation levels (nmol per gwt).
Measurement of MDA
To determine the amount of oxidative damage in the spinal cord, levels of MDA were measured spectrophotometrically by the method of Uchiyama and Mihara,14 which follows changes in malondialdehyde precursors in tissue through the use of the thiobarbituric acid test. To 0.5 ml of tissue homogenate, 3 ml of 1% H3PO4 and 1 ml 0.6 thiobarbituric acid aqueous solutions were added, then stirred and heated in a boiling water bath for 45 min. After cooling, 4 ml of n-butanol was added, and the mixture was shaken. The butanol layer was separated by centrifugation and the absorbance of the butanol layer was measured spectrophotometrically at 535 and 520 nm. The difference between the two optical densities was a measure of the thiobarbituric acid value. A calibration curve was drawn by using 1,1,3,3-tetramethoxypropane, and results were presented as nanomoles of MDA per gram of wet tissue (g.w.t).
Measurement of GSH
GSH was assayed by reacting with O-phthaldialdehyde (10 mg/10 ml methanol) according to the modified method of Lee and Chung.15 Briefly, tissue was homogenized in ice-cold 6% perchloric acid at 4 °C. Supernatant was collected after centrifugation at 4000 × g for 10 min at 4 °C.
An aliquot of the supernatant was allowed to react with O-phthaldialdehyde for 5 min. Pure reduced GSH was used as standard for calibration. GSH samples were measured by using spectrofluorimetry (excitation at 345 nm and emission at 425 nm).
Assessment of caspase-3 activity
All spinal cord samples were homogenized in lysis buffer (Tris-NaCl-EDTA-protease inhibitors) by using glass-dounce homogenizer. The homogenate was centrifuged to pellet protein at 13 000 r.p.m. at 4 °C for 15 min. Supernatants were run on SDS-PAGE after heating at 65 °C for 3 min by standard procedures using the Mini Protean II system (Bio-Rad, Hercules, CA, USA). After electrophoresis, proteins were transferred to a PVDF membrane (Bio-Rad) using the Trans-Blot Semi-Dry system (Bio-Rad) at 20 V for 30 min. The membranes were blocked in Roti-Block reagent (Roth, Germany) with gentle shaking at RT for 1 h. After washing with tris-buffered saline (TBS) (20 mM Tris, pH 7.5, 150 mM NaCl), blots were incubated with anti active product of caspase-3 (that react only with 17 kDa subunit, Chemicon, AB3623) antibody (1:200) for 2 h. Blots were washed three times with tris-buffered saline and tween 20 (TBS-T) (20 mM Tris, pH 7.5, 150 mM NaCl, 0.05% Tween 20) and incubated with secondary goat anti-rabbit antibody conjugated with alkaline phosphatese (1:30 000) for 1 h. After washing with TBS-T(3 ×) and with TBS only (1 × ), a color reaction was performed in the dark with NBT/BCIP alkaline phosphatase substrate tablets (Sigma fast tablet) under agitation for 10–30 min, until development of a black–purple color. The membranes were then scanned, and the integrated optical density of the resulting bands was determined by Fluor-S-Multi-Imager (Bio-Rad, München, Germany) and given as arbitrary units.
Statistical analysis
Statistical calculations were performed by using Excel (Microsoft, Redmond, WA, USA) and SPSS (SPSS, Inc., Chicago, IL, USA). The normality of distributions of data was evaluated with the Shapiro–Wilk test and was found abnormal (P<0.05). The results are expressed as mean±s.e.m. Differences between the group means were analyzed for significance using the Mann–Whitney U-tests. In all cases, a P-value of <0.05 was considered to be statistically significant.
Results
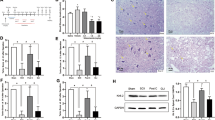
As expected, IR caused spinal cord damage as indicated by increased levels of MDA, decreased GSH contents and the occurrence of measurable caspase-3 activity. MDA levels were found to be significantly increased in the IR group compared with the control group, IR and mel+IR groups (Figure 1). Control group GSH levels were found to be significantly decreased (P<0.05). GSH levels were found to be significantly increased in the mel+IR group (Figure 2).
Spinal cord tissue (L3–L4) malondialdehyde (MDA) levels in the experimental groups. Control (C), Melatonin (Mel), Ischemia/reperfusion (IR), Melatonin+Ischemia/reperfusion (Mel+IR), gram wet tissue (gwt). The values are shown as mean±s.e.m. Different letters indicate significant difference between the bars (P<0.05).
Spinal cord tissue (L3–L4) reduced glutathione (GSH) levels in the experimental groups. Control (C), Melatonin (Mel), Ischemia/reperfusion (IR), Melatonin+Ischemia/reperfusion (Mel+IR), gram wet tissue (gwt). The values are shown as mean±s.e.m. Different letters indicate significant difference between the bars (P<0.05).
In the normal control of spinal cord tissue, we failed to find any measurable active p17 fragment of caspase-3 (Figures 3a and b). However, in the spinal cord tissue of the IR group, a marked activation of caspase-3 occurred (to 1.2 AU: P<0.05).
(a) Spinal cord tissue (L3–L4) optical density of active caspase-3 protein from western blotting. Control (C), Melatonin (Mel), Ischemia/reperfusion (IR), Melatonin+Ischemia/reperfusion (Mel+IR). The values are shown as mean±s.e.m. Different letters indicate significant difference between the bars (P<0.05). (b) Western blot analysis of rat spinal cord tissue (L3–L4). 1. Control, 2. Melatonin, 3. Ischemia reperfusion, 4. Melatonin+Ischemia reperfusion, 5. Marker. The experiment was repeated three times, with similar results.
Melatonin application reduced the IR-induced caspase-3 activation to 0.1 AU (P<0.05).
Discussion
Although oxygen is critical for life and for maintenance of metabolic processes, reactive metabolites of oxygen may be toxic to cells. In particular, the cellular damage that occurs secondary to ischemia may be exacerbated by the sudden re-oxygenation into tissues during onset of the reperfusion period, triggering free radical cascades that overwhelm endogenous free radical scavengers. Here we show that the IR-induced alterations of rat spinal cord involve an activation of caspase-3, as an indicator of apoptosis, an increase in lipid peroxidation (assayed by measuring MDA) and an exhaustion of the endogenous free radical scavenger, GSH.
Melatonin is a free radical scavenger, an antioxidant and an immunomodulatory agent. In addition to many normal physiological roles of melatonin, the neuroprotective properties of this pineal gland hormone have been investigated in neurodegenerative diseases and injuries. Investigations of the chemical properties of melatonin, both in vivo and in vitro, indicate that melatonin and its metabolites are potent antioxidants that directly scavenge both reactive oxygen species (ROS) and reactive nitrogen species (RNS) in the cells.4, 6, 16 Besides, melatonin stimulates many antioxidant enzymes. The antioxidant properties of melatonin are connected with its neuroprotective activity in several degenerative disorders. One of the causes of neurodegenerative damage in the nervous system is oxidative injury, which results from an imbalance between free radical formation and antioxidative mechanisms.17 The efficacy of melatonin in the inhibition of the oxidative stress was estimated in various neurodegenerative disorders. The mechanisms involved in melatonin protection against ischemia–reperfusion damage seem to be mainly mediated by its antioxidant properties. A direct relationship between the protection by melatonin and its antioxidant properties was found by Sinha et al.18 who besides the decreased lesion volume and the improvement of neurological deficits, reported prevention by melatonin of lipid peroxidation and glutathione reduction induced by ischemia. In the other experimental model, Kılıc et al.19 confirmed the recovery by melatonin of Akt phosphorylation levels, also reporting the increase in the antiapoptotic Bcl-XL protein expression and the decrease in caspase-3 activity.
In the present study, we used a powerful antioxidant agent, melatonin, to examine its effects on the SCI-induced oxidative injury and apoptosis. It is well known that the formation of MDA, an end product of lipid peroxidation, is a major indicator of oxidative injury.1, 5, 20 In the current study, SCI caused significant elevations in MDA levels in spinal cord tissues demonstrating oxidative stress, while caspase-3 activity was also increased. It was reported that, in animal models of SCI, melatonin has been found highly effective in reducing the level of lipid peroxidation following injury, and the effects are attributed to its antioxidative actions.20, 21 Furthermore, our results are also in agreement with the study of Samantaray et al.1 who demonstrated that melatonin significantly decreased caspase-3 activity in SCI animals. Melatonin prevents cell damage and death. As both calpain and caspase-3 activities are known to participate in cell death, the implication is that reduction in their activities by melatonin may have protected neurons in the SCI following injury.1
Reduced GSH has an important role in cell metabolism, differentiation, proliferation and apoptosis, and as a result disturbances in its homeostasis are implicated in the ethology and/or progression of a number of human diseases, including neurodegenerative diseases and injuries. Several reports indicate that tissue injury induced by various stimuli is coupled with GSH depletion. Melatonin is a well-known direct, free radical scavenger and neutralizer of a number of reactive oxygen and nitrogen species.
In conclusion, because melatonin has almost no toxicity, is available and easily administered, clinical studies of its potential for protecting SCI may be warranted.
DATA ARCHIVING
There were no data to deposit.
References
Samantaray S, Sribnick EA, Das A, Knaryan VH, Matzelle DD, Yallapragada AV et al. Melatonin attenuates calpain upregulation, axonal damage and neuronal death in spinal cord injury in rats. J Pneal Res 2008; 44: 348–357.
Ayala A, Munoz MF, Arguelles S . Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014; 2014: 360438.
Draper HH, Hadley M . Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 1990; 186: 421–431.
Allegra M, Reiter RJ, Tan DX, Gentile C, Tesoriere L, Livrea MA . The chemistry of melatonin's interaction with reactive species. J Pineal Res 2003; 34: 1–10.
Erten SF, Kocak A, Ozdemir I, Aydemir S, Colak A, Reeder BS . Protective effect of melatonin on experimental spinal cord ischemia. Spinal Cord 2003; 41: 533–538.
Rodriguez C, Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V et al. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res 2004; 36: 1–9.
Jain A, Bhatnagar M . Melatonin-a ‘magic biomolecule’. Ann Neurosci 2007; 14: 1–13.
Li M, Ona VO, Chen M, Kaul M, Tenneti L, Zhang X et al. Functional role and therapeutic implications of neuronal caspase-1 and-3 in a mouse model of traumatic spinal cord injury. Neuroscience 2000; 99: 333–342.
Rao RV, Hermel E, Castro-Obregon S, del Rio G, Ellerby LM, Ellerby HM et al. Coupling endoplasmic reticulum stress to the cell death program. Mechanism of caspase activation. J Biol Chem 2001; 276: 33869–33874.
Friedlander RM . Mechanisms of disease: Apoptosis and caspases in neurodegenerative diseases. N Engl J Med 2003; 348: 1365–1375.
Keane RW, Kraydieh S, Lotocki G, Bethea JR, Krajewski S, Reed JC et al. Apoptotic and anti-apoptotic mechanisms following spinal cord injury. J Neuropathol Exp Neurol 2001; 60: 422–429.
Ventimiglia R, Lau LF, Kinloch RA, Hopkins A, Karran EH, Petalidis LP et al. Role of caspases in neuronal apoptosis. Drug Dev Res 2001; 52: 515–533.
Zivin JA, Degirolami U . Spinal-Cord Infarction - Highly Reproducible Stroke Model. Stroke 1980; 11: 200–202.
Mihara M, Uchiyama M . Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 1978; 86: 271–278.
Lee AY, Chung SS . Contributions of polyol pathway to oxidative stress in diabetic cataract. FASEB J 1999; 13: 23–30.
Bonnefont-Rousselot D, Collin F, Jore D, Gardes-Albert M . Reaction mechanism of melatonin oxidation by reactive oxygen species in vitro. J Pineal Res 2011; 50: 328–335.
Emerit J, Edeas M, Bricaire F . Neurodegenerative diseases and oxidative stress. Biomed Pharmacother 2004; 58: 39–46.
Sinha K, Degaonkar MN, Jagannathan NR, Gupta YK . Effect of melatonin on ischemia reperfusion injury induced by middle cerebral artery occlusion in rats. Eur J Pharmacol 2001; 428: 185–192.
Kilic U, Kilic E, Reiter RJ, Bassetti CL, Hermann DM . Signal transduction pathways involved in melatonin-induced neuroprotection after focal cerebral ischemia in mice. J Pineal Res 2005; 38: 67–71.
Ersahin M, Ozdemir Z, Ozsavci D, Akakin D, Yegen BC, Reiter RJ et al. Melatonin treatment protects against spinal cord injury induced functional and biochemical changes in rat urinary bladder. J Pineal Res 2012; 52: 340–348.
Fujimoto T, Nakamura T, Ikeda T, Takagi K . Potent protective effects of melatonin on experimental spinal cord injury. Spine 2000; 25: 769–775.
Acknowledgements
This work was supported by a grant from The Scientific and Research Project Unit of Inonu University (BAP-2009004), Turkey.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Aydemir, S., Dogan, D., Kocak, A. et al. The effect of melatonin on spinal cord after ischemia in rats. Spinal Cord 54, 360–363 (2016). https://doi.org/10.1038/sc.2015.204
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2015.204
This article is cited by
-
Effects of ginsenoside Rb1 on spinal cord ischemia-reperfusion injury in rats
Journal of Orthopaedic Surgery and Research (2019)
-
Studies on protection against ischemia reperfusion injury after SCI
Spinal Cord (2016)